Home / EMMA Capnograph
EMMA Capnograph

EMMA® Capnograph
Portable Real-time Capnography with Bluetooth® Connectivity
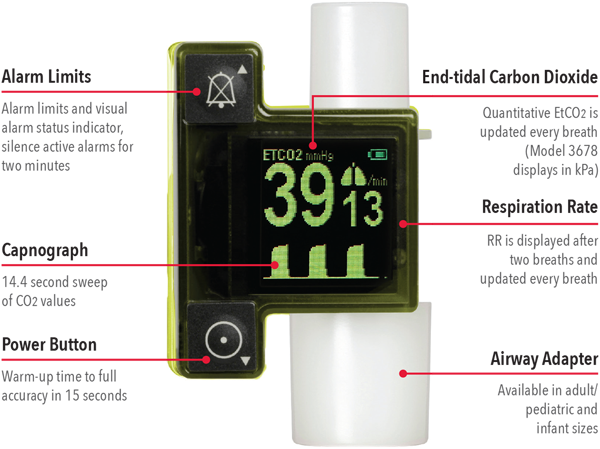
- Immediate results — minimal warm-up time, with fully accurate end-tidal carbon dioxide (EtCO2) and respiration rate (RR) measurements and continuous EtCO2 waveform displayed within 15 seconds1
- Airway management — real-time EtCO2 waveforms help clinicians ensure proper endotracheal tube placement,2 assess the depth and effectiveness of compressions, and recognise the return of spontaneous circulation3
- Small, lightweight, and tetherless — convenient during short-term EtCO2 monitoring of adult, paediatric, and infant patients
- Designed to fit easily onto a breathing circuit — flexible use across multiple care areas, including pre-hospital, emergency medicine, operating room, intensive care unit, long-term acute care, and patient transport
- Connectivity — integrates with Masimo monitors to provide a larger waveform display, measurement trending, and seamless transfer of patient data to the electronic medical record
Features
Features
- Easy to maintain with no routine calibration required
- Small, portable design easily stores in a crash cart
Kit

EMMA Kit*
- EMMA Bluetooth (mmHg) PN 4271
- EMMA Bluetooth (kPa) PN 4270
- EMMA Capnograph (mmHg) PN 3639
- EMMA Capnograph (kPa) PN 3678
Accessories

EMMA Airway Adapter
- Adult/Paediatric
- Box of 25
- PN 17448

EMMA Airway Adapter
- Infant
- Box of 10
- PN 17449
References:
- 1.
Operator's Manual.
- 2.
Link MS, et al. Circulation. 2015; 132(suppl 2): S444–S464.
- 3.
Neumar RW et al. Circulation. 2010;122:S729-S767.
* In order for the EMMA Kit to provide readings, either of the listed airway adapters is required. Kit includes EMMA, pouch, and lanyard.
RESOURCES
EMMA with Bluetooth is not licensed for sale in Canada.
For professional use. See instructions for use for full prescribing information, including indications, contraindications, warnings, and precautions.
PLCO-001898/ PLM-10667B-0518

