News & Media-2013
Home / 2013
2013
NEWS & MEDIA:


Children's Hospital St. Elisabeth in Neuburg/Donau Installs Masimo Patient SafetyNet™ System for Advanced Care and Oversight of General Ward Patients
Neuburg, Germany & Irvine, California – December 19, 2013 – Children's Hospital St. Elisabeth in Neuburg, and Masimo (NASDAQ: MASI) today announced that the hospital installed the largest Patient SafetyNet™ system in Germany. Patient SafetyNet is a remote monitoring and clinician notification system shown to keep patients safer, enabling a 65% reduction in rapid response team activations and 48% reduction in ICU transfers.1
The installation includes Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry technology, proven by more than 100 independent and objective studies and used on an estimated 100 million patients a year in leading hospitals worldwide, as well as rainbow® Acoustic Monitoring (RAM™), a breakthrough respiration rate (RRa) measurement used with a cloth adhesive sensor worn on the neck that allows clinicians to noninvasively and continuously assess patients' breathing, facilitating earlier detection of respiratory compromise and patient distress.
Carmen Bauer, head nurse of ward 15 at the Children's Hospital, stated: "With Masimo Patient SafetyNet covering 40 beds on two general children wards, we are now able to feel absolutely safe for our patients. We have observed that it's especially beneficial for our most vulnerable patients, who potentially suffer from respiratory depression. And, our staff has been extremely satisfied with the system."
St. Elisabeth joins a growing list of hospitals around the world using Patient SafetyNet, which leverages the performance of Masimo SET® pulse oximetry. Compared to other pulse oximeters during patient motion and low perfusion, Masimo SET® provides measurements when other pulse oximeters cannot, significantly reduces false alarms (specificity), and accurately detects true alarms (sensitivity)2,3 that can help clinicians identify a deteriorating patient. Most importantly, Masimo SET® pulse oximetry is the only technology clinically proven to improve patient outcomes by helping clinicians reduce retinopathy of prematurity (ROP)4 in neonates, screen newborns for critical congenital heart disease (CCHD),5,6 reduce ventilator weaning time and arterial blood gas measurements in the ICU,7 and save lives and costs while reducing rapid response activations and intensive care unit transfers on the general floor.3
Patient SafetyNet can help ensure patients' safety by noninvasively and continuously measuring and tracking their underlying physiological conditions and detecting changes or abnormalities in vital sign measurements that may signal declining health status in real-time. When changes occur in the measured values, which may indicate deterioration in the patient's condition, the system automatically sends wireless alerts directly to clinicians – prompting a potentially lifesaving response to the patient's bedside.
1 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. Available online
2 Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers".J Clin Anesth. 2012 Aug;24(5):385-91.
3 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2.
4 Castillo A, et al. Acta Paediatr. 2011 Feb.;100(2):188-92.
5 de-Wahl Granelli A., et al. BMJ. 2009 Jan 8;338.
6 Ewer A, et al. Health Technol Assess. 2012;16(2):1-184.
7 Durbin, et al. Critical Care Medicine. 2002 Aug.;30(8): 1735 to 1740.
About St. Elisabeth Children's Hospital in Neuburg
St. Elisabeth was built out of three hospitals with 321 beds in total and has a history starting in 1840. The children's hospital has been awarded twice for excellence in Children Care. For more information, visit http://www.kliniken-st-elisabeth.de
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that Masimo Patient SafetyNet can help keep patients safer by noninvasively, continuously measuring and tracking their underlying physiological condition to help hospitals avoid preventable patient deaths and injuries associated with failure to rescue events, risks related to our assumptions of the repeatability of clinical results obtained, and risks related to the system's ability to significantly decrease traumatic critical events and costly ICU transfers to help improve patient outcomes and reduce costs; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at https://www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Thomas Bauch
Kinderklinik St. Elisabeth
Phone: +49 8431 541190
Email: thomas.bauch@kliniken-st-elisabeth.de
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


Happy Holidays from Masimo!
Irvine, California – December 16, 2013 – From the launch of Root, an intuitive patient monitoring and connectivity platform destined to transform patient care, to multiple product awards and a growing catalog of positive clinical studies, this year has been one of great achievements. As we honor this very special time of the season, it is with humble gratitude and a heart-felt desire to give something back to a few of the organizations that share our vision for a better world, in your name. Simply respond with an email to charity@masimo.com specifying your charity choice from the list below and we will donate $10 in your name:
 |
- Amnesty International
- CARE
- Clinton Foundation
- Doctors Without Borders
- Huntington's Disease Society of America
- Make-a-Wish Foundation
- March for Babies
- Masimo Foundation for Ethics, Innovation, and Competition in Healthcare
- Opportunity International
- PATH
- Patient Safety Movement Foundation
- SOS Children's Villages
- Swan Foundation
- Tiyatien Health
- UNICEF
- United Way
- World Vision
We are excited to kick off the New Year with the second-annual Patient Safety, Science & Technology Summit, Jan. 11-13, with the Joint Commission for Transforming Healthcare as a co-convener, and again featuring former President Bill Clinton as the keynote. We are grateful for the opportunity to have transformed healthcare and we thank each of you for your support in helping us to improve the lives of clinicians and patients we diligently serve. Here's to a New Year full of boundless possibilities!
Masimo Mission Statement
Improving patient outcomes and reducing cost of care by taking noninvasive monitoring to new sites and applications.®
Masimo Guiding Principles
- Remain faithful to your promises and responsibilities.
- Thrive on fascination and accomplishment and not on greed and power.
- Make each day as fun as possible.
- Strive to make each year better than the year before, both personally and for the Team.
- Do what is best for patient care.
NOTE: Only e-mails sent to charity@masimo.com from official Livewire members will be processed. Please also include any comments or suggestions you might have that will help us to better fulfill our mission and adhere to our guiding principles.


Study Shows Noninvasive SpCO® Helps Clinicians Detect Occult Carbon Monoxide Poisoning
Irvine, California – December 10, 2013 – Masimo (NASDAQ: MASI) announced today that a new clinical study recently published in the Emergency Medicine Journal shows that Masimo's noninvasive carboxyhemoglobin (SpCO®) from rainbow® Pulse CO-Oximetry™ helped clinicians identify 23% more patients with carbon monoxide (CO) poisoning who presented to the emergency department (ED) with headaches.1
CO poisoning is the leading cause of death resulting from accidental poisoning worldwide. Lack of timely diagnosis can delay treatment or, worse, patients discharged with an incorrect diagnosis can be re-exposed to CO. While CO poisoning happens year-round in all climates, the incidents typically increase during colder months due to the use of fossil fuel-burning heating appliances. CO poisoning accounts for an estimated 50,000 ED visits in the U.S. annually.2 Headaches are the most common symptom of CO poisoning – others include dizziness, nausea/vomiting, confusion, fatigue, chest pain, shortness of breath, and loss of consciousness. CO poisoning often is often misdiagnosed and attributed to other illnesses such as the flu. Failure to diagnose CO poisoning can have disastrous consequences for patients and potentially other family members of affected households.3
In their prospective study patients presenting to the ED between Feb. 1 and March 31, 2011, researchers Nilay Zorbalar, Murat Yesilaras, and Ersin Aksay at Izmir Tepecik Research and Educational Hospital in Izmir, Turkey, used a Masimo Rad-57™ Pulse CO-Oximeter to noninvasively assess SpCO on 482 patients who complained of headaches.
Patients whose initial SpCO measurement was greater than 10% underwent a venous blood draw for laboratory determination of invasive carboxyhemoglobin (COHb) measurement. If a patient's invasive COHb level was greater than 10%, they were diagnosed with CO poisoning. Of the 482 patients presenting with headaches who were screened with SpCO measurement, 38 had a mean SpCO value of over 10%, 31 (6.4% of the study population) of which had elevated COHb confirmed by laboratory determination. As a screening measurement, SpCO had a positive predictive value of 82% for CO poisoning. Of the 31 true CO poisoning cases, 24 (77%) were suspected and seven (23%) were not suspected. Therefore, SpCO screening was responsible for helping the ED clinicians detect the 23% of cases that would be considered "occult," or unsuspected, CO poisoning.
The investigators concluded: "CO poisoning should be kept in mind in patients presenting to the ED with a headache. SpCO is an effective screening tool to detect CO poisoning in these patients."
1 Zorbalar N, Yesilaras M, Aksay E. "Carbon monoxide poisoning in patients presenting to the emergency department with a headache in winter months." Emerg Med J Published online ahead of print Oct. 15, 2013
2 Hampson N., Weaver L. "Carbon Monoxide Poisoning: A New Incidence for an Old Disease." Undersea Hyperb Med 2007;34:163-168.
3 Hampson N., Piantadosi C., Thom S., Weaver L. "Practice Recommendations in the Diagnosis, Management, and Prevention of Carbon Monoxide Poisoning," American Journal of Respiratory and Critical Care Medicine, Vol. 186, No. 11 (2012), pp. 1095-1101.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SpCO will provide an accurate and effective noninvasive method of screening for CO poisoning, risks related to our belief that SpCO offers an effective front-line tool for first responders and emergency departments, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at https://www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57, Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.



Masimo and Newborn Foundation Jointly Announce Mobile Health Initiative to Reduce Global Newborn Mortality
Newborn Foundation's BORN Project Launches with New iSpO2Rx Mobile Technology for Early Detection of Health Conditions in Newborns in Low-Resource Settings
Irvine, California & Washington, D.C., December 6, 2013 – Masimo (NASDAQ: MASI) today announced the launch of iSpO2 TM Rx Pulse Oximeter with M-LNCS TM connector, enabling adhesive sensor use on newborns for accurate and cost-effective screening with mobile devices in low-resource settings. iSpO2 Rx features Masimo SET® technology – shown through studies to be the most accurate pulse oximetry during challenging conditions and proven to help clinicians identify life-threatening conditions in newborns. iSpO2 Rx, available outside the U.S., provides accurate oxygen saturation (SpO2), pulse rate (PR), and perfusion index (PI) results from an iPhone, iPad, and iPod touch, and soon on select mobile devices for Android™.
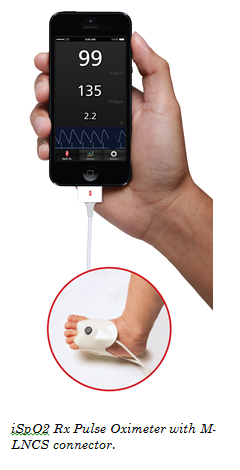 |
iSpO2 Rx, announced ahead of the mHealth Summit in Washington, D.C., Dec. 8-11, is the lynchpin of an ongoing collaboration with the Newborn Foundation, and marks the debut of the BORN Project – Birth Oximetry Routine for Newborns – the first global health initiative to reduce infant mortality through earlier detection of illness, disease and congenital birth defects through access to mobile-enabled technology that measures blood-oxygen levels in newborns. Until now, available pulse oximeters in low-resource settings have been inaccurate, lacked the ability to use adhesive sensors required for screening newborns, and were unable to simply and wirelessly transfer test results.
Globally, about 3.3 million newborns die within the first month of life, with neonatal infection, sepsis, pneumonia and birth defects among the major killers, according to the World Health Organization. The BORN Project was developed to help reduce neonatal mortality, a critical Millennium Development Goal, by deploying accessible and effective mobile technology targeting early detection of several of the major causes of newborn death. Clinical studies have shown that pulse oximetry screening can help detect conditions such as pneumonia, early-onset sepsis, neonatal infection and pulmonary hypertension,1 in addition to congenital heart defects.
"This simple, noninvasive check of oxygen levels in newborns is among the most effective health measures that can be deployed to reduce newborn mortality," said Annamarie Saarinen, co-founder and chairman of the Newborn Foundation. "We just saw how valuable this is, firsthand, while in the Philippines during Typhoon Haiyan. The mHealth Summit marks a historic opportunity to share this initiative and new technology that is finally available to babies in low-resource healthcare environments through mHealth innovation. We're very excited to be partnering with Masimo and public health to deploy this technology and advance its use as part of routine newborn care."
"From a public health perspective, neonatal infection, sepsis and pneumonia can all be treated more effectively if the conditions are detected early," said Darshak Sanghavi, MD, fellow and managing director, Engelberg Center for Health Care Reform at the Brookings Institution and associate professor of pediatrics at the University of Massachusetts Medical School. "Incorporating routine pulse oximetry as a universal 'vital sign' in newborns could be an important global life-saving tool."
"Through the BORN Project, Masimo is eager to help clinicians – especially those working in low-resource areas of the world – gain access to Masimo SET iSpO2 Rx as the most effective, affordable pulse oximeter that is accurate in challenging conditions of patient movement and low perfusion to help save the lives of newborns," said Masimo founder and CEO Joe Kiani.
More about iSpO2 Rx
iSpO2 Rx utilizes the same Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry technology proven by more than 100 independent and objective studies and used on more than 100 million patients a year in leading hospitals worldwide, including eight of the top 10 on the U.S. News & World Report Best Hospitals Honor Roll (2013-2014). Compared to other pulse oximeters, Masimo SET® provides measurements specifically validated during patient motion and low perfusion, both common in newborns. Moreover, Masimo SET®pulse oximetry has been shown to help clinicians reduce retinopathy of prematurity (ROP)2 in neonates, and effectively screen newborns for congenital heart disease.3,4 iSpO2 Rx also features a downloadable and upgradeable iSpO2 Rx App, ensuring clinicians have the latest telemedicine technologies at their fingertips at all times. iSpO2 Rx can store and email up to 12 hours of measurement history in a global standard .CSV file format. iSpO2 Rx with the M-LNCS connector is compatible with Masimo adhesive and reusable sensors, and are uniquely designed to offer the best pulse oximetry performance for use in neonatal, infant, pediatric, and adult patients.
iSpO2 Rx also features a downloadable and upgradeable iSpO2 Rx App, ensuring clinicians have the latest telemedicine technologies at their fingertips at all times. iSpO2 Rx can store and email up to 12 hours of measurement history in a global standard .CSV file format. iSpO2 Rx with the M-LNCS connector is compatible with Masimo adhesive and reusable sensors, and are uniquely designed to offer the best pulse oximetry performance for use in neonatal, infant, pediatric, and adult patients.
iPad, iPhone, and iPod touch are trademarks of Apple Inc., registered in the U.S. and other countries.
1 Ewer A. Review of pulse oximetry screening for critical congenital heart defects in newborn infants. Curr Opin Cardiol 2013;28:92–6.
2 Castillo A, et al. Acta Paediatr. 2011 Feb.;100(2):188-92.
3 de-Wahl Granelli A., et al. BMJ. 2009 Jan 8;338.
4 Ewer A, et al. Health Technol Assess. 2012;16(2):1-184.
About Newborn Foundation
The Newborn Foundation is an international non-profit working specifically to leverage health IT and medical technologies to improve access and outcomes while reducing disparities for newborns. The organization has been integral in the policy development, adoption and implementation of technologies for early detection, intervention and care of the youngest patients, including universal newborn heart screening as a public health initiative.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure -Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors related to the new iSpO2 Rx, including our belief in the breakthrough ability of Masimo SET® pulse oximetry to measure-through motion and low perfusion; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at https://www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Jing Zhang
Newborn Foundation
Phone: (651) 414-1095
Email: jing@newborncoalition.org
Mike Drummond
Masimo Corporation
Phone:(949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SedLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium. All other trademarks, service marks, trade names, logos and icons, registered or not, are the property of third parties as named/indicated.



ACUTRONIC Medical Systems Integrates Masimo SET® Pulse Oximetry into fabian HFO Neonatal Critical Care Ventilators for Improved Patient Outcomes
Zurich, Switzerland & Irvine, California – November 22, 2013 – Masimo (NASDAQ: MASI) announced today that ACUTRONIC Medical Systems AG has integrated Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry into ACUTRONIC's fabian HFO Neonatal Critical Care Ventilator for improved patient outcomes.
The fabian HFO, the world's first dedicated Neonatal Ventilator to offer Masimo SET® pulse oximetry, provides continuous display of newborn oxygenation status. Instead of using two separate devices to collect ventilation and oxygenation patient data, clinicians now simply attach the pulse oximetry sensor via USB interface to the fabian HFO to display oxygenation, perfusion index and pulse rate values on the ventilator's colour touchscreen. This combined technology platform will optimize patient assessments and improve workflow, allowing clinicians to spend more valuable time tending to patients.
"The integration of Masimo SET® within ACUTRONIC's fabian HFO ventilator is providing caregivers the ability to better assess their patients' oxygenation status, even during challenging conditions of patient motion and low perfusion," said Roland Hotz, President. "With our fabian HFO neonatal critical care ventilator, we offer clinicians leading technology on a highly versatile platform specifically designed for the most vulnerable of patient populations."
Masimo's President of Worldwide OEM Business & Corporate Development, Rick Fishel stated, "The synergies realized between our companies and products support Masimo's mission to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. We are very pleased that Masimo's SET® Pulse Oximetry platform is a key component in ACUTRONIC's new neonatal ventilators."
Masimo SET® pulse oximetry has been shown to virtually eliminate false alarms1 and increase a clinician's ability to detect life-threatening events2 – helping to substantially contribute to improved patient outcomes and patient safety. The clinical accuracy of Masimo SET® pulse oximetry has also been shown to help clinicians significantly reduce retinopathy of prematurity (ROP),3-5 screen for critical congenital heart disease (CCHD) in newborns,6-8 reduce oxygen overdose9,10 and medical errors,11,12 and save lives in post-surgical floors, recovery,2 labor and delivery rooms,13 and ICUs.14
1 Shah N, Ragaswamy H, Govindugari K, Estanol L. "Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers." Journal of Clinical Anesthesia. 2012 (10.1016/j.jclinane.2011.10.012) https://www.sciencedirect.com
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Impact-of-Pulse-Oximetry-Surveillance-on-Rescue
3 Chow L.C., Wright K.W., Sola A.; CSMC Oxygen Administration Study Group. "Can Changes in Clinical Practice Decrease the Incidence of Severe Retinopathy of Prematurity in Very Low Birth Weight Infants?" Pediatrics. 2003 Feb;111(2):339-45.
4 Clinical Practice and SpO2 Technology in the Prevention of ROP in VLBW Infants. Castillo AR, Deulofeut R, Sola A. Presented at Pediatric Academic Societies Annual Meeting May 5-8, 2007.
5 Oxygen as a neonatal health hazard: call for détente in clinical practice. Sola A., Rogido, Marta, Deulofeut, Richard. Acta Paediatrica 2007; 96:801-812.
6 de Wahl Granelli A, Wennergren M, Sandberg K, et al. Impact of pulse oximetry screening on detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ 2009; 338: a3037.
7 Noninvasive Peripheral Perfusion Index as a Possible Tool for Screening for Critical Left Heart Obstruction. Granelli, AD, Ostman-Smith, I. Acta Paediatrica 2007; 96:1455-1459.
8 Screening for Duct-Dependent Congenital Heart Disease with Pulse Oximetry: A Critical Evaluation of Strategies to Maximize Sensitivity. Granelli AD, Mellander M, Sunnegardh J,Sandberg K, Ostman-Smith I. Acta Paediatr 2005; 94:1590-6.
9 Avoiding Hyperoxemia During Neonatal Resuscitation: Time to Response to Different SpO2 Monitors. Baquero H, Alviz R, Sola A. Presented at Pediatric Academic Societies Annual Meeting May 5-8, 2007.
10 More Reliable Oximetry Reduces the Frequency of Arterial Blood Gas Analyses and Hastens Oxygen Weaning after Cardiac Surgery. Durbin CG, Rostow SK. Crit Care Med 2002; 30(8): 1735-1740
11 Advantages of New Technology Pulse Oximetry with Adults in Extremis. Durbin CG, Rostow SK. Anesth Analg 2002; 94: S81-S83
12 Pulse Oximetry Performance Can Affect Caregiver Time Utilization. Durbin CG, Rostow SK. Anesthesiology 2000;93(3A): A556.
13 Baquero H, Alviz R, Castillo A, Neira F, Sola A. "Avoiding Hyperoxemia During Neonatal Resuscitation: Time To Response Of Different SpO2 Monitors." Acta Paed April 2011 Vol. 100, Issue 4, pp 515-518. Published online https://onlinelibrary.wiley.com/doi/full/10.1111/j.1651-2227.2010.02097.x
14 Goldstein MR, Martin GI, Sindel BD, Furman GI, Ochikubo C, Yand L. SatSeconds Alarm Management Misses Short Desaturations Common to Periodic Breathing and Infantile Apnea. Pediatric Research 2001;49(4):400A/2296.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
About Masimo
ACUTRONIC is a Swiss company developing, manufacturing and distributing high tech ventilation solutions for worldwide NICU's/PICU's, ICU's and Intensive Care Transportation. We are the leading partner for hospitals when it comes to the most critical areas of respiratory care: Neonatalogy/Pediatrics, Jet Ventilation and Difficult Airway Management. Our innovative products simplify the busy clinical routine for caregivers and increase the safety of patients. With over 30 years of experience and together with our global network of clinical experts, we keep challenging ourselves to continuously improve and set standards – like the 'fabians', the most silent neo-ventilation concept in the marketplace today. We strive every day to provide the technologically best solutions that are needed for the most medically challenging patients. ACUTRONIC. Ventilation Beyond Limits.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of published studies, internal clinical studies and side-by-side clinical evaluations; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at https://www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Maurizio Ginepro
ACUTRONIC Medical Systems
Phone: +41-44-729 70 80
Email: m.ginepro@acutronic-medical.ch
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.



Masimo Receives 2013 Zenith Award at the American Association of Respiratory Care Congress
Irvine, California – November 18, 2013 – Masimo (NASDAQ: MASI), the inventor of Pulse CO-Oximetry™ and Measure-through Motion and Low Perfusion™ pulse oximetry, announced today that it has received the prestigious Zenith Award for equipment and service excellence from the American Association of Respiratory Care (AARC) at the 59th Annual AARC Congress in Anaheim, Calif.
With the 2013 Zenith Award, Masimo has achieved AARC's top industry recognition honoring respiratory care equipment and pharmaceutical manufacturers for quality and service excellence. More than 400 companies were eligible for only one of six Zenith Award honors. Award winners are chosen by the association's membership—more than 52,000 respiratory care professionals—based upon the quality of equipment and/or supplies, accessibility and helpfulness of sales personnel, as well as the company's responsiveness, service record, truth in advertising, and overall support of the respiratory care profession.
"Masimo typifies the qualities represented in the criteria and, because of this, we salute you and your employees," said AARC Executive Director/CEO Thomas J. Kallstrom.
"We are honored by the appreciation of so many AARC members who have once again recognized us with the Zenith Award," said Joe Kiani, founder & CEO of Masimo. "This recognition is especially meaningful and gratifying because it comes from the clinicians who use our products every day."
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at https://www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, sSpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SedLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


New Published Clinical Study Shows Masimo PVI® Helps Clinicians Assess Fluid Responsiveness in Ventilated Patients in the Early Phase of Septic Shock
Irvine, California – November 5, 2013 – Masimo (NASDAQ: MASI) announced today a new study in the Journal of Critical Care demonstrates that Masimo's noninvasive PVI® can be used to help clinicians accurately assess fluid responsiveness in mechanically ventilated patients in the early phase of septic shock in the emergency room.1
Early, goal-directed treatment of sepsis is particularly important and is recommended by the Surviving Sepsis guidelines, but these efforts can be complicated if dynamic invasive monitoring tools are not readily available.
In the study conducted at Centre Hospitalier de Belfort-Montbeliard in Belfort, France, Marc Feissel, M.D., and a team of researchers evaluated 31 mechanically ventilated and sedated adult patients with septic shock in whom volume expansion was planned. Investigators used a Masimo Radical-7 Pulse CO-Oximeter®, which automatically calculated and displayed PVI. Intervention consisted of infusing 8 mL/kg of hydroxylethyl starch over a 20-minute period. Before and after intervention, researchers recorded PVI and measured the aortic velocity-time integral (VTIao) using transthoracic echocardiography.
Sixteen patients were classified as responders; 15 as nonresponders. Responders were defined as patients who increased their VTIao by 15% or higher after fluid infusion. Mean PVI values before intervention were significantly higher in responders vs nonresponders (30% ± 9% vs 8% ± 5%, P < .001). PVI values before intervention were correlated with percent changes in VTIao induced by intervention (r=0.82, r2 = 0.67; P < .001).
A PVI threshold value of 19% distinguishes responders from nonresponders to fluid challenge with a sensitivity of 94% and a specificity of 87% in mechanically ventilated patients in the early phase of septic shock.
The researchers concluded that PVI is a "feasible and interesting method to predict fluid responsiveness in the early phase septic shock patients."
Investigators also noted, "Training emergency department physicians in the use and interpretation of PVI is quick, easy, and inexpensive;" "removing the need for central arterial catheter placement in the emergency department represents a considerable time gain and avoids an additional puncture site that could be a potential port of entry for bacteria;" and, "optimizing hemodynamic status as early as possible, that is, in the emergency department, could help avoid progression to multi-organ failure, thereby reducing morbidity and mortality in patients with severe sepsis or septic shock."
PVI is a dynamic noninvasive measurement of changes over the respiratory cycle that provides clinicians with a continuous, easy-to-use, and cost-effective measure for assessing whether patients will benefit from fluid administration – enabling personalized and goal-directed fluid therapy.2-6
Steve Barker, M.D., Ph.D., Interim Chief Medical Officer of Masimo, stated: "This study demonstrates the practical and cost-effective advantage of PVI, particularly for physicians for whom time and ease of use are of the essence. The findings also add to the growing body of evidence that shows the efficacy of PVI across a wide range of patient populations."
1 Feissel M., Kalakhy R., Banwarth P., Badie J., Pavon A., Faller J., Quenot J.; "Plethysmographic variation index predicts fluid responsiveness in ventilated patients in the early phase of septic shock in the emergency department: A pilot study." J Crit Care 2013 Oct; 28(5): 634-639.
2 Loupec T., Nandoumgar H., Frasca D., Petitpas F., Laksiri L., Baudouin D., Debaene B., Dahyot-Fizelier C., Mimoz O. "Pleth Variability Index Predicts Fluid Responsiveness in Critically-Ill Patients." Crit Care Med 2011 Feb;39(2):294-9. http://journals.lww.com/ccmjournal/Abstract/2011/02000/Pleth_variability_index_predicts_fluid.8.aspx.
3 Zimmerman M., Feibicke T., Keyl C., Prasser C., Moritz S., Graf B., and Wiesenack C. "Accuracy of Stroke Volume Variation Compared with Pleth Variability Index to Predict Fluid Responsiveness in Mechanically-ventilated Patients Undergoing Major Surgery." Eur J Anaesthesiol. 2010 Jun;27(6):555-61. https://www.ncbi.nlm.nih.gov/pubmed/20035228.
4 Feissel M., Kalakhy R., Badie J., Robles G., Faller J., Teboul JL. "Plethysmography Variability Index: A New Fluid Responsiveness Parameter." Presented at the 29th International Symposium on Intensive Care and Emergency Medicine (ISICEM) Annual Meeting, March 25, 2009, Brussels, Belgium. http://ccforum.biomedcentral.com/articles/10.1186/cc7369.
5 Cannesson M., Desebbe O., Rosamel P., Delannoy B., Robin J., Bastien O., Lehot JJ. "Pleth variability Index to Monitor the Respiratory Variations in the Pulse Oximeter Plethysmographic Waveform Amplitude and Predict Fluid Responsiveness in the Operating Theatre." Br J Anaesth. 2008 Aug;101(2):200-6. https://www.ncbi.nlm.nih.gov/pubmed/18522935.
6 Forget P, Lois F, De Kock M. "Goal-Directed Fluid Management Based on the Pulse Oximeter-Derived Pleth Variability Index Reduces Lactate Levels and Improves Fluid Management." Anesth Analg. 2010 Oct;111(4):910-4. Published online http://journals.lww.com/anesthesia-analgesia/pages/default.aspx.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using Masimo PVI, risks related to our belief that PVI is an easy-to-use and cost-effective measure for assessing whether patients will benefit from fluid administration, risks related to our assumptions that PVI enables personalized and goal-directed fluid therapy, and that PVI is a preferred noninvasive indicator of fluid responsiveness in children, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57, Rad-8, Rad-5, Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SedLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo Announces Japan Launch of iSpO2 Pulse Oximeter for iPhone, iPad, and iPod touch
Telemedicine Innovation Offers Measure-Through Motion, Low Perfusion SET® Pulse Oximetry for Real-Time or Remote Patient and Data Management
Irvine, California – October 31, 2013 – Masimo (NASDAQ: MASI) today announced availability in Japan of iSpO2, the world's first Measure-through Motion and Low Perfusion™ pulse oximeter for iPhone, iPad, and iPod touch.
 Masimo iSpO2 pulse oximeter now available in Japan. |
More than ever, people the world over desire access to high quality health data through mobile devices. Now, with the same Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry used on an estimated 100 million-plus patients per year in leading hospitals worldwide, iSpO2 provides accurate, real-time oxygen saturation (SpO2), pulse rate (PR), and perfusion index (PI) results on compatible iOS devices.
iSpO2 can trend, store and email up to 12 hours of measurement history in a global standard .CSV file format, allowing patients and clinicians to share data remotely.
iSpO2 – extremely lightweight at just 232 grams or about 0.5 pounds – also offers:
- Multiple user interface language capability
- Instant software upgrades via the iSpO2 App
- Visible pulse rate via the Pleth waveform, demonstrating the unique ability of Masimo SET® to measure-through motion and low perfusion.
The performance of Masimo SET® pulse oximetry is proven by more than 100 independent and objective studies and thousands of clinical evaluations to accurately monitor blood oxygen saturation even in the most challenging conditions, including patient movement and low peripheral perfusion. The pulse oximetry standard-of-care at leading hospitals worldwide – including by eight of the top 10 hospitals on the U.S. News & World Report Best Hospitals Honor Roll (2013-2014) – Masimo SET® virtually eliminates false alarms1 and increases a clinician's ability to detect life-threatening events.2
"The availability of iSpO2 in Japan is another example of Masimo's commitment to fulfilling real global demand for mobile technologies that help users access data about themselves, whenever and wherever they are," said Tetsuro Maniwa, President of Masimo Japan.
For more information about iSpO2 and other Masimo technologies, visit https://www.masimo.co.jp
1 Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers".J Clin Anesth. 2012 Aug;24(5):385-91.
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Impact-of-Pulse-Oximetry-Surveillance-on-Rescue
Apple, iPad, iPhone, iPod and iPod touch are registered trademarks of Apple Inc. "Made for iPod®," "Made for iPhone®," and "Made for iPad®" mean that an electronic accessory has been designed to connect specifically to iPod, iPhone, or iPad, respectively, and has been certified by the developer to meet Apple performance standards. Apple is not responsible for the operation of this device or its compliance with safety and regulatory standards. Please note that the use of this accessory with iPod, iPhone, or iPad may affect wireless performance.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors related to the new iSpO2, including our belief in the breakthrough ability of Masimo SET® pulse oximetry to measure-through motion and low perfusion; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at https://www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57, Rad-8, Rad-5, Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SedLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium. All other trademarks, service marks, trade names, logos and icons, registered or not, are the property of third parties as named/indicated.


Masimo to Report Third Quarter 2013 Financial Results after Market Close on Wednesday, October 30
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
Irvine, California, October 18, 2013 – -- Masimo (NASDAQ: MASI) announced today that it will release third quarter 2013 financial results for the period ended September 28, 2013, after the market closes on Wednesday, October 30, 2013. The conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at https://www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 87025552. After the live webcast, the call will be available on Masimo's website through November 30, 2013. In addition, a telephonic replay of the call will be available through November 13, 2013. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 87025552.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. And in 2012, Masimo acquired the assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
# # #
Contact: Eli Kammerman
(949) 297-7077
ekammerman@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Multiple New Clinical Studies Presented at the American Society of Anesthesiologists Annual Meeting Show Benefits of RAM & PVI
Irvine, California – October 17, 2013 – Masimo (NASDAQ: MASI) announced today that four new clinical studies evaluating Masimo noninvasive patient monitoring technologies were presented at the largest gathering of anesthesiologists in the world, the American Society of Anesthesiologists (ASA) Annual Meeting in San Francisco. The following studies highlight the positive clinical outcomes and patient safety impact of Masimo's unique rainbow® noninvasive measurement technologies, including RAM™ acoustic respiration rate (RRa™) and PVI®.
RAM™
At Stanford University Medical School in Menlo Park, Calif., Dr. Pedro Tanaka et al., evaluated RRa and capnography during sedation. The investigators reported: "Of the respiratory pauses detected by a single method, acoustic monitoring had the highest percentage of true positives and the lowest percentage of false positives." They concluded: "When compared to clinical observation or capnography, rainbow® acoustic monitoring (RAM) may provide the best method for detection of respiratory pause during procedures requiring sedation because it has acceptable accuracy for detection of respiratory pause with a low rate of false alarms."1
At Loma Linda University Medical Center in Loma Linda, Calif., Dr. Richard Applegate et al., found that compared to nasal cannula capnometry (Covidien Capnostream® 20), RAM RRa "detected more true events and had fewer false alarms," adding, "Compared to nasal cannula capnometry, acoustic respiratory monitoring may be a superior monitor of respiration during procedural sedation."2
At Tokyo Women's Medical University, School of Medicine, in Tokyo, Japan, Dr. Tomoko Fukada, et al., evaluated RAM (RRa), capnometry (CAP), and thoracic impedance pneumography (IMP) and compared them with visual 1-minute chest movement inspection. Investigators concluded: "Continuous respiration rate assessments with RRa correlated well with visual inspection, even for obese patients and patients with respiration rate ≤10 per minute. CAP measurements also showed good correlation with visual chest movement inspection, but the equipment was often removed by some patients because of displeasure or an inserted gastric tube. IMP measurements were inaccurate because of electrode positioning and patient movement and shivering. Therefore, RRa was the most accurate and noninvasive method to monitor respiration rate for detecting apnea, bradypnea, and airway obstruction."3
PVI® with Masimo SET® Pulse Oximetry
At University of California Irvine Medical Center in Irvine, Calif., Cecilia Canales, M.P.H., et al., evaluated whether goal-directed fluid optimization based on PVI can be used reliably intra-operatively in patients undergoing moderate-risk surgery. Investigators concluded, "Goal-directed fluid optimization based on respiratory variation in the pulse oximeter waveform is feasible, and may help to standardize intraoperative fluid management."4
1 Tanaka P, Drover D, Tanaka M. "Detection of Respiratory Pauses by Clinical Observation: Capnography and Acoustic Monitoring" Proceedings of the American Society of Anesthesiologists, October 16, 2013. San Francisco. A5033
2 Applegate R, Lenart J, Malkin M, Macknet M. "Respiratory Pause Detection and False Alarms from Capnography and Acoustic Monitoring in Procedure Related Sedation" Proceedings of the American Society of Anesthesiologists, October 16, 2013. San Francisco. A5027
3 Fukada T, Uenaka Y, Tsuchiya Y, Iwakiri H, Nomura M. "Clinical Evaluation on Acoustic Respiration Rate (RRa™) in PACU Compared With the Conventional Monitoring System" Proceedings of the American Society of Anesthesiologists, October 12, 2013. San Francisco. A1180
4 Canales C, Lee C, Khanh-Van L, Hanacek C, Natalia N, Nguyen C, Le A, Boud R, Rinehart J, Cannesson M. "Feasibility of Goal-Directed Fluid Management Based on Monitoring of Pleth Variability Index (PVI)" Proceedings of the American Society of Anesthesiologists, October 13, 2013. San Francisco. A2211
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®), PVI®, and acoustic respiration rate (RRa™) contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at https://www.sec.gov. . Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57, Rad-8, Rad-5, Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SeDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Multicenter Study Shows Clinical Value of rainbow® Acoustic Monitoring (RAM™)
Researchers Conclude RAM Has Potential to Increase Pediatric Patient Safety
Irvine, California – October 8, 2013 – Masimo (NASDAQ: MASI) announced today a new study in Pediatric Anesthesia demonstrating Masimo's noninvasive, continuous rainbow® Acoustic Monitoring (RAM™) had similar accuracy, yet better patient tolerance compared to capnography (nasal cannula) in post-surgical pediatric patients.1
The study, which compared the performance of rainbow® Acoustic Monitoring to capnography in a pediatric patient population, adds to the growing body of evidence that shows the efficacy of RAM (including accuracy, precision, and better patient tolerance) in adult and pediatric patient populations.2,3,4,5
RAM is a breakthrough respiration rate (RRa) measurement used with a cloth adhesive sensor worn on the neck that allows clinicians to noninvasively and continuously assess patients' breathing, facilitating earlier detection of respiratory compromise and patient distress.
In the multicenter study at Cincinnati Children's Hospital Medical Center, University of Arizona Medical Center, and Children's Medical Center at Dallas, researchers found, "When compared to nasal capnography, RRa showed good agreement and similar accuracy and precision but was better tolerated in post-surgical pediatric patients."
The difference in bias and precision between the two test methods was not statistically significant (p=0.41).
Investigators concluded: "Acoustic monitoring has the potential to increase the safety of pediatric patients by providing a reliable and accurate method for the continuous monitoring of respiration rate."
Study Results
| Comparison of Methods | Bias +/- SD (bpm) | Limits of Agreement (bpm) |
|---|---|---|
| RRa with Capnography (Oridion Capnostream 20) | -0.3 ± 3.5 | -7.3 to 6.6 |
| RRa with Reference | -0.1 ± 2.5 | -5.0 to 5.0 |
| Capnography (Oridion Capnostream 20) with Reference | 0.2 ± 3.4 | -6.8 to 6.72 |
Six of the 40 patients in the study immediately removed the nasal cannula and would not permit it to be reapplied (and were not included in data collection); nine of the remaining 34 patients removed it prior to study completion (but were included in data collection). Only one patient removed the RRa sensor after 80 minutes of monitoring time.
The reliability of capnography was 92% of total monitoring time and the reliability of RRa (used with a Masimo Rad-87™ bedside monitor) was 90% of monitoring time; the difference was not statistically significant (p=0.54). Total duration of monitoring time and average per patient was 2,650 minutes and 76 minutes for capnography and 2,849 minutes and 83 minutes for RRa.
Steve Barker, M.D, Ph.D., Interim Chief Medical Officer of Masimo, stated: "This study shows that because RAM is as accurate and is better tolerated than capnography, more post-surgical pediatric patients are likely to remain monitored longer, potentially increasing patient safety and improving outcomes. Additionally, where capnography is not a standard of care – such as PACUs and general floors – RAM, due to its ease of use may enable continuous respiration rate monitoring which could improve patient safety considerably."
1 Patino M., Redford D.T., Quigley T.W., Mahmoud M., Kurth C.D., Szmuk P. Accuracy of acoustic respiration rate monitoring in pediatric patients. Pediatric Anesthesia. 2013 Sep 3.
2 Macknet M.R., Kimball-Jones P.L., Applegate R.L., Martin R.D., Allard M.W. Accuracy and Tolerance of a Novel Bioacoustic Respiratory Sensor in Pediatric Patients. Anesthesiology 2007; 107: A84.
3 Ramsay M. Usman M. Lagow E. Mendoza M. Untalan E. De Vol E. The Accuracy, Precision and Reliability of Measuring Ventilatory Rate and Detecting Ventilatory Pause by Rainbow Acoustic Monitoring and Capnometry. Anesthesia & Analgesia. July 2013
4 Mimoz et al. Accuracy of respiratory rate monitoring using a non-invasive acoustic method after general anaesthesia Br. J. Anaesth. (2012) 108(5): 872-875 first published online February 8, 2012
5 Goudra B.G., Penugonda L. Monitoring Respiration in Upper GI Endoscopy Anesthesia. Proceedings of the 2011 Annual Meeting of the American Society of Anesthesiologists. A246.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results and performance of Masimo rainbow® Acoustic Monitoring; our belief that the breakthrough acoustic respiration rate monitoring capabilities of Masimo's proprietary RRa technology will provide sufficient sensitivity and specificity to measure respiratory rate in a variety of patients and monitoring conditions, enabling clinicians to detect and treat respiratory compromise and patient distress earlier; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at https://www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57, Rad-8, Rad-5, Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.



EMMA™ Palm Size Capnograph Earns 2013 EMS World Top Innovation Award
Irvine, California – October 4, 2013 – Masimo(NASDAQ: MASI) announced today that its EMMA™ Capnograph with waveform display earned a prestigious EMS World Top Innovation Award, another accolade for a powerful, portable device, that offers immediate waveform capnography, end-tidal carbon dioxide and respiration rate at your fingertips.
 Masimo EMMA™ Capnograph. |
EMMA was one of 65 products entered as a new, innovative product in emergency medical services. A committee narrowed the field to finalists, which were selected for in-depth evaluation at the recent EMS World Expo.Of those, 20 were selected as Top New Product Innovations to be featured in the December 2013 issue of EMSWorld Magazine, as well as on EMSWorld.com, and profiled in the January 2014 print issue.
Launched in July 2013, EMMA Capnograph offers clinicians assessment of end-tidal carbon dioxide (EtCO2) and respiration rate, as well as assisting in recognition of return to spontaneous circulation, for a variety of clinical settings, including emergency medicine and transport, ORs, ICUs, patient rooms, and clinics. Rugged, water-resistant and operational in first-responder and other clinically challenging conditions, EMMA Capnograph displays and monitors respiratory rate and EtCO2 continuously with full accuracy within 15 seconds when connected to a patient's breathing circuit. Powered by two standard AAA batteries, EMMA's portability allows for easy use during cardiopulmonary resuscitation (CPR) and intubation in multiple points of care.
Previously, sister product EMMA Capnometer won a JEMS Hot Products Award from the Journal of Emergency Medical Services at the EMS Today Conference and Exposition.
"We are honored that our EMMA Capnograph continues to earn high praise from all corners of the EMS industry," said Jon Coleman, Masimo President, Masimo Worldwide Sales, Professional Services and Medical Affairs. "The EMS World Top Innovation Award is particularly uplifting given the intensely competitive field of EMS devices."
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to the performance of EMMA and its ability to provide end-tidal CO2 assessment during cardiopulmonary resuscitation (CPR) and intubation for all patients in all clinical settings in just a few seconds; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at https://www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SedLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.



Rochester General Health System Installs Masimo Patient SafetyNet™ for Advanced Care and Oversight of Patients
Installation Is Part of the System-Wide Conversion to Masimo SET® Pulse Oximetry
Irvine, California – September 13, 2013 – Masimo (NASDAQ: MASI) today announced that Rochester General Health System and its 528-bed flagship Rochester General Hospital have installed the Masimo Patient SafetyNet™ system, a remote monitoring and clinician notification system shown to dramatically reduce rapid response activations, transfers to intensive care units, and deaths related to opioid-induced respiratory depression.1
Rochester General joins a growing list of prominent health systems around the world using Patient SafetyNet, which combines the performance of Masimo SET® pulse oximetry, the enabler of reliable monitoring in the general ward, with ventilation monitoring and wireless clinician notification. Patient SafetyNet can can help ensure patients' safety by noninvasively and continuously measuring and tracking their underlying physiological conditions and changes that signal declining health status in real-time. When changes occur in the measured values, which may indicate deterioration in the patient's condition, the system automatically sends wireless alerts directly to clinicians – prompting a potentially lifesaving response to the patient's bedside.
"Masimo provides us with the advanced technologies we need to meet our high standards for patient care," said Claire Aloan, MS, RRT-NPS, FAARC, Director of Respiratory Care Services for the Rochester General Health System. "Patient SafetyNet offers us the ability to respond to patients faster. And Masimo SET® pulse oximetry – because of its clinical accuracy – gives us confidence that we're responding to true alarms. This speed and accuracy allows us to improve patient outcomes
The installation at Rochester General Hospital took place after an extensive evaluation process resulting in the organization's standardization to Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry. The performance of Masimo SET® pulse oximetry is proven by more than 100 independent and objective studies and thousands of clinical evaluations. Masimo SET® is trusted by clinicians to safely monitor more than 100 million patients each year and is used hospital-wide by eight of the top 10 hospitals on the U.S. News & World Report Best Hospitals Honor Roll (2013-2014). Compared to other pulse oximeters during patient motion and low perfusion, Masimo SET® provides measurements when other pulse oximeters cannot, dramatically reduces false alarms (specificity), and accurately detects true alarms (sensitivity)2,3 that can indicate a deteriorating patient. Most importantly, Masimo SET® pulse oximetry has been shown to improve patient outcomes by helping clinicians reduce retinopathy of prematurity (ROP)4 in neonates, screen newborns for critical congenital heart disease (CCHD),5,6 reduce ventilator weaning time and arterial blood gas measurements in the ICU,7 and save lives and costs while reducing rapid response activations and intensive care unit transfers on the general floor.3
"Rochester General Health System has made patient safety a top priority," said Joe Kiani, Founder and CEO of Masimo. "We are honored to partner with a healthcare facility that is committed to help protect patients, improve outcomes, and reduce costs."
1 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. Available online
2 Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers".J Clin Anesth. 2012 Aug;24(5):385-91.
3 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Impact-of-Pulse-Oximetry-Surveillance-on-Rescue
4 Castillo A, et al. Acta Paediatr. 2011 Feb.;100(2):188-92.
5 de-Wahl Granelli A., et al. BMJ. 2009 Jan 8;338.
6 Ewer A, et al. Health Technol Assess. 2012;16(2):1-184.
7 Durbin, et al. Critical Care Medicine. 2002 Aug.;30(8): 1735 to 1740.
About Rochester General Health System
Serving the community of Greater Rochester and beyond, Rochester General Health System (RGHS) has earned national recognition for excellence in a variety of clinical specialties. RGHS is comprised of eight clinically integrated affiliates delivering comprehensive healthcare services with superior quality, safety and patient satisfaction. Rochester General Hospital (RGH), the health system's flagship affiliate, is a 528-bed tertiary care facility that treats more Monroe County patients than any other hospital. RGH, home to the fourth largest cardiac center in New York State, has been recognized nine times as being among the nation's 100 Top Cardiovascular Hospitals. According to the 2013 report from CareChex®, a division of The Delta Group, RGH ranks first in New York for Major Cardiac Surgery (#4 in the nation) and Heart Attack Treatment, #2 in New York for Overall Medical Care and #5 statewide for Overall Surgical Care. For more information, please visit https://www.rochesterregional.org/
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that all neonatal patients at Rochester General Hospital will be cared for using the most technologically and clinically-advanced noninvasive patient monitoring solutions available; risks related to our belief that Masimo Patient SafetyNet can help keep patients safer by noninvasively, continuously measuring and tracking their underlying physiological condition to help hospitals avoid preventable patient deaths and injuries associated with failure to rescue events; risks related to our belief that Masimo SET virtually eliminates false alarms and increases a clinician's ability to detect life-threatening events; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at https://www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Barbara McManus
Rochester General Health System
Phone: (585) 922-4763
Email: barbara.mcmanus@rochestergeneral.org
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SedLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.

![]()

Saint Alphonsus Regional Medical Center Installs Masimo Patient SafetyNet™ System for Improved Oversight of Patients
Installation Is Part of the Hospital-Wide Conversion to Masimo SET® Pulse Oximetry
Boise, Idaho & Irvine, California – September 6, 2013 – Saint Alphonsus Regional Medical Center, a 387-bed facility renowned for its use of pioneering technologies, and Masimo (NASDAQ: MASI) today announced that the hospital has installed Masimo Patient SafetyNet™ in patient rooms on orthopedic, general surgery, and neurology floors. Patient SafetyNet is a remote monitoring and clinician notification system clinically shown to keep patients safer by enabling remote continuous monitoring of the patient's physiology, including oxygenation and pulse rate, which has led to saved lives, reductions in rapid response activations and transfers to intensive care units.1
Patient SafetyNet combines the performance of Masimo SET® pulse oximetry with ventilation monitoring and wireless clinician notification. Patient SafetyNet provides an extra measure in patients' safety by noninvasively and continuously measuring and tracking changes in oxygenation that can signal a declining health status. When changes occur in the measured values, the system sends wireless alerts directly to clinicians – prompting a response to the patient's bedside.
"We are glad to have this extra layer of safety for our patients," said Adrianne Presnell, nurse manager of the orthopedic unit at Saint Alphonsus Regional Medical Center. "It really benefits our more vulnerable patients, who could potentially suffer from respiratory depression."
Masimo SET® is trusted by clinicians to safely monitor more than 100 million patients each year and is used hospital-wide by eight of the top 10 hospitals on the U.S. News & World Report Best Hospitals Honor Roll (2013-2014). Compared to other pulse oximeters, during patient motion and low perfusion, Masimo SET® provides accurate measurements when other pulse oximeters cannot, dramatically reduces false alarms (increased specificity), and accurately detects true alarms (increased sensitivity)2,3 that can indicate a deteriorating patient. Most importantly, Masimo SET® pulse oximetry has been shown to improve patient outcomes by helping clinicians reduce retinopathy of prematurity (ROP)4 in neonates, screen newborns for critical congenital heart disease (CCHD),5,6 reduce ventilator weaning time and arterial blood gas measurements in the ICU,7 and save lives and costs while reducing rapid response activations and intensive care unit transfers on the general floor.3
"We are honored to partner with Saint Alphonsus Regional Medical Center, a healthcare facility with a track record of adopting leading-edge technologies to help protect patients, improve outcomes, and reduce costs," said Joe Kiani, Founder and CEO of Masimo.
1 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. Available online
2 Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers".J Clin Anesth. 2012 Aug;24(5):385-91.
3 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Impact-of-Pulse-Oximetry-Surveillance-on-Rescue
4 Castillo A, et al. Acta Paediatr. 2011 Feb.;100(2):188-92.
5 de-Wahl Granelli A., et al. BMJ. 2009 Jan 8;338.
6 Ewer A, et al. Health Technol Assess. 2012;16(2):1-184.
7 Durbin, et al. Critical Care Medicine. 2002 Aug.;30(8): 1735 to 1740.
About Saint Alphonsus Regional Medical Center
Saint Alphonsus is a 387-licensed bed regional medical center serving people throughout the Northwest. As a not-for-profit medical center, Saint Alphonsus reinvests profits back into the community and works to improve the health and well-being of those we serve by emphasizing care that is patient-centered, innovative and community based. Saint Alphonsus focuses on providing services in a spiritual, healing environment, and is renowned for its state-of-the-art digital environment and pioneering technologies; award-winning clinical services; and the region's only Level II Trauma Center. Saint Alphonsus is part of Saint Alphonsus Health System, a four-hospital, 714-bed integrated healthcare system serving the full range of the health and wellness needs of the people in southwestern Idaho, eastern Oregon and northern Nevada. Saint Alphonsus Health System is a member of Trinity Health. For information about Saint Alphonsus, visit https://www.saintalphonsus.org.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that all patients at Saint Alphonsus Regional Medical Center will be cared for using the most technologically and clinically-advanced noninvasive patient monitoring solutions available; risks related to our belief that Masimo Patient SafetyNet can help keep patients safer by noninvasively, continuously measuring and tracking their underlying physiological condition to help hospitals avoid preventable patient deaths and injuries associated with failure to rescue events, risks related to our assumptions of the repeatability of clinical results obtained, and risks related to the system's ability to significantly decrease traumatic critical events and costly ICU transfers to help improve patient outcomes and reduce costs; risks related to our belief that Masimo SET virtually eliminates false alarms and increases a clinician's ability to detect life-threatening events; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at https://www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Jennifer Krajnik
Saint Alphonsus Regional Medical Center
Phone: (208) 367-6762
Email: jennkraj@sarmc.org
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57, Rad-8, Rad-5, Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SedLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.



Firat University Hospital Installs Masimo Patient SafetyNet™ System for Improved Oversight of NICU Patients
Elazig, Turkey & Irvine, California – August 29, 2013 – Firat University Hospital, one of the Eastern Turkey's leading health centers, and Masimo (NASDAQ: MASI) today announced that the hospital has deployed Masimo Patient SafetyNet™, clinically shown to help improve patient outcomes and save money.1
Firat joins a growing list of leading healthcare systems around the world using Patient SafetyNet, which can help ensure patient safety by noninvasively and continuously measuring and tracking a patient's underlying physiological conditions and detect changes or abnormalities that signal declining health status in real-time. When changes occur in the measured values, which may indicate deterioration in the patient's condition, the system automatically sends wireless alerts directly to clinicians – prompting a potentially lifesaving response to the patient's bedside. Patient SafetyNet also has been clinically shown to reduce preventable and costly rescue events and transfers to intensive care units.1
"We have found that Masimo Patient SafetyNet offers the best system when it comes to noninvasive bedside monitoring technologies," said Dr. Erdal Taskin, associate professor of neonatology at Firat University Hospital. "Masimo SET® Pulse Oximetry and the Patient SafetyNet System reduced the number of clinical alarms in our neonatal intensive care unit by about 70 percent within the first five days of installation. Our workload in the NICU has been reduced because of far fewer false alarms, which we believe will help improve patient outcomes."
The installation of Patient SafetyNet at Firat University Hospital took place after an extensive evaluation process resulting in the organization's standardization to Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry. The performance of Masimo SET® pulse oximetry is proven by more than 100 independent and objective studies and thousands of clinical evaluations. Masimo SET® is trusted by clinicians to safely monitor more than 100 million patients each year and is used hospital-wide by eight of the top 10 U.S. hospitals on the U.S. News & World Report Best Hospitals Honor Roll (2013-2014). Compared to other pulse oximeters during patient motion and low perfusion, Masimo SET® provides measurements when other pulse oximeters cannot, dramatically reduces false alarms (specificity), and accurately detects true alarms (sensitivity)2,3 that can indicate a deteriorating patient. Most importantly, Masimo SET® pulse oximetry is the only technology clinically proven to improve patient outcomes by helping clinicians reduce retinopathy of prematurity (ROP)4 in neonates, screen newborns for critical congenital heart disease (CCHD),5,6 reduce ventilator weaning time and arterial blood gas measurements in the ICU,7 and save lives and costs while reducing rapid response activations and intensive care unit transfers on the general floor.3
"Firat University Hospital has earned a reputation for delivering high-quality patient care in this tremendously important region of the world," said Joe Kiani, Founder and CEO of Masimo. "Through its embrace of advanced technology and medical services, it has distinguished itself as one of the best hospitals in the area. We are happy and honored to be partners with Firat, and look forward to helping it improve patient outcomes through Masimo's advanced patient monitoring technologies."
1 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. Available online
2 Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers".J Clin Anesth. 2012 Aug;24(5):385-91.
3 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Impact-of-Pulse-Oximetry-Surveillance-on-Rescue
4 Castillo A, et al. Acta Paediatr. 2011 Feb.;100(2):188-92.
5 de-Wahl Granelli A., et al. BMJ. 2009 Jan 8;338.
6 Ewer A, et al. Health Technol Assess. 2012;16(2):1-184.
7 Durbin, et al. Critical Care Medicine. 2002 Aug.;30(8): 1735 to 1740.
About Firat University Hospital
Firat University Hospital is one of the Eastern Turkey's leading health centers, equipped with leading-edge technology and medical services, including a coronary intensive care unit, hemodialysis center, and a nuclear medicine unit. The hospital also is on the forefront of fertility diagnosis and treatment. Visit our website at https://www.firat.edu.tr/en
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that Masimo Patient SafetyNet can help keep patients safer by noninvasively, continuously measuring and tracking their underlying physiological condition to help hospitals avoid preventable patient deaths and injuries associated with failure to rescue events, risks related to our assumptions of the repeatability of clinical results obtained, and risks related to the system's ability to significantly decrease traumatic critical events and costly ICU transfers to help improve patient outcomes and reduce costs; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at https://www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.



Douglasville Nursing and Rehabilitation Center Installs Masimo Patient SafetyNet™ System for Improved Patient Monitoring
Installation Is Part of the Organization-Wide Conversion to Masimo SET® Pulse Oximetry
Douglasville, Georgia & Irvine, California – August 22, 2013 – Douglasville Nursing and Rehabilitation Center and Masimo (NASDAQ: MASI) today announced that the hospital has deployed Masimo Patient SafetyNet™, clinically shown to help improve patient outcomes and save money.1
Patient SafetyNet can help ensure patients' safety by noninvasively and continuously measuring and tracking their underlying physiological conditions and detect changes that signal declining health status in real-time. When changes occur in the measured values, which may indicate deterioration in the patient's condition, the system automatically sends wireless alerts directly to clinicians – prompting a potentially lifesaving response to the patient's bedside. Patient SafetyNet also has been clinically shown to reduce preventable, costly rescue events and transfers to intensive care units.1
"Whenever we hear the alarm we can respond to it right away and get to the room fast," Vannie Lee, director of respiratory therapy at Douglasville Nursing and Rehabilitation Center, said of Patient SafetyNet. "It gives us a heads up on what's going on with our patients when we're not at the bedside. Patient SafetyNet has saved many patients' lives here. I don't know what I'd do without it."
The installation of Patient SafetyNet at Douglasville Nursing and Rehabilitation Center is part of the healthcare organization's standardization to Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry. The performance of Masimo SET® is proven by more than 100 independent and objective studies and thousands of clinical evaluations. Masimo SET® is trusted by clinicians to safely monitor more than 100 million patients each year and is used hospital-wide by eight of the top 10 U.S. hospitals on the U.S. News & World Report Best Hospitals Honor Roll (2013-2014). Compared to other pulse oximeters during patient motion and low perfusion, Masimo SET® provides measurements when other pulse oximeters cannot, dramatically reduces false alarms (specificity), and accurately detects true alarms (sensitivity)2,3 that can indicate a deteriorating patient. Most importantly, Masimo SET® pulse oximetry has been shown to reduce ventilator weaning time and arterial blood gas measurements in the ICU,4 and save lives and costs while reducing rapid response activations and intensive care unit transfers on the general floor.3
"We are excited to partner with Douglasville Nursing and Rehabilitation Center to help address its need for continuous patient monitoring," said Jon Coleman, President, Masimo Worldwide Sales, Professional Services and Medical Affairs. "With the addition of Patient SafetyNet and Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry, Douglasville Nursing and Rehabilitation Center is armed with powerful technologies to help improve patient outcomes and reduce costs of care."
1 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. Available online
2 Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers".J Clin Anesth. 2012 Aug;24(5):385-91.
3 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Impact-of-Pulse-Oximetry-Surveillance-on-Rescue
4 Durbin, et al. Critical Care Medicine. 2002 Aug.;30(8): 1735 to 1740.
About Douglasville Nursing and Rehabilitation Center
Douglasville Nursing and Rehabilitation Center, LLC, located in Douglasville, Georgia, proudly serves our community's long term care and rehabilitation needs. We are committed to providing loving excellence in senior care and you can be assured that your loved ones will be in good hands – each receiving individual and specialized care to meet their health care needs from our compassionate and clinically trained professionals. Douglasville Nursing and Rehabilitation Center, LLC is both Medicare and Medicaid certified, and participates in several managed care plans including BlueCross BlueShield, United Healthcare, Humana, and Coventry. Visit https://www.douglasvillerehabcenter.com/
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that all Douglasville Nursing and Rehabilitation Center patients will be cared for using Masimo Patient SafetyNet; risks related to our belief Patient SafetyNet can help keep patients safer by noninvasively, continuously measuring and tracking their underlying physiological condition to avoid preventable deaths and injuries associated with failure to rescue events, and risks related to the system's ability to significantly decrease traumatic critical events and costly ICU transfers to help improve patient outcomes and reduce costs; risks related to our believe Masimo SET virtually eliminates false alarms and increases a clinician's ability to detect life-threatening events; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at https://www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SedLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.



Pathways Nursing & Rehabilitation Center Standardizes to Masimo SET® Pulse Oximetry
Specialty Healthcare Facility Deploys Breakthrough Measure-Through Motion and Low Perfusion Pulse Oximetry Technology
Niskayuna, New York & Irvine, California – August 16, 2013 – Masimo (NASDAQ: MASI) and Pathways Nursing & Rehabilitation Center, specializing in on-site medical and rehabilitation treatments to optimize recovery for sub acute, traumatic brain injury, ventilator-dependent, and pediatric patients, jointly announce that Pathways has converted to Masimo SET® pulse oximetry and sensor technologies, the standard-of-care at leading health organizations worldwide.
Pathways' conversion to Masimo SET® pulse oximetry is in keeping with the healthcare organization's dedication to excellence in care and delivering the best possible results for its patients.
The performance of Masimo SET® pulse oximetry is proven by more than 100 independent and objective studies and thousands of clinical evaluations. Masimo SET® is trusted by clinicians to safely monitor more than 100 million patients each year and is used hospital-wide by eight of the top 10 U.S. hospitals on the U.S. News & World Report Best Hospitals Honor Roll (2013-2014). Compared to other pulse oximeters during patient motion and low perfusion, Masimo SET® provides measurements when other pulse oximeters cannot, dramatically reduces false alarms (specificity), and accurately detects true alarms (sensitivity)1,2 that can indicate a deteriorating patient. Most importantly, Masimo SET® pulse oximetry has been shown to be the only proven technology to improve patient outcomes by helping clinicians reduce retinopathy of prematurity (ROP)3 in neonates, screen newborns for critical congenital heart disease (CCHD),4,5 reduce ventilator weaning time and arterial blood gas measurements in the ICU,6 and save lives and costs while reducing rapid response activations and intensive care unit transfers on the general floor.2
"We have this one little girl we affectionately call 'wild woman'," says Sandy Dykens, Director of Respiratory at Pathways. "She was weaned off a ventilator here, and she is very active – always grabbing things and moving around. Because she is so active, the alarm on her old pulse oximeter kept going off with a false oxygen desaturation, and we kept running to her bedside to make sure she was all right."
"With Masimo you get a truer reading and far fewer false alarms, so now when we do hear alarms, we don't risk missing something that really needs our attention," Dykens adds. "'Wild woman' is still very active. But when we go to her bedside these days, it's to see her playing, not rushing in to expect to see her crashing. And her wellbeing is so much better because of it. She can be active without staff interrupting her play to check on her sensors. She has more of a normal childhood. We're so appreciative of this."
"We are happy to have forged this great partnership with Pathways, which like other great healthcare organizations is demonstrating its commitment to patient safety and care by taking advantage of our breakthrough measure-through motion and low perfusion pulse oximetry technology," said Masimo Founder and CEO Joe Kiani. "We value this relationship with Pathways, and together look forward to helping their skilled and dedicated staff meet and exceed their patient-care needs."
1 Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers".J Clin Anesth. 2012 Aug;24(5):385-91.
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Impact-of-Pulse-Oximetry-Surveillance-on-Rescue
3 Castillo A, et al. Acta Paediatr. 2011 Feb.;100(2):188-92.
4 de-Wahl Granelli A., et al. BMJ. 2009 Jan 8;338.
5 Ewer A, et al. Health Technol Assess. 2012;16(2):1-184.
6 Durbin, et al. Critical Care Medicine. 2002 Aug.;30(8): 1735 to 1740.
About Pathways Nursing & Rehabilitation Center
Pathways Nursing and Rehabilitation Center is a unique and comprehensive rehabilitation center serving TBI, Pediatric and Sub Acute patients. Located in the beautiful Mohawk Valley, Pathways is a 112-bed facility, providing on-site medical and rehabilitation treatments to optimize recovery for sub acute, TBI, Ventilator-dependent and pediatric patients. Our caring professionals provide comprehensive individual care plans to ensure the highest quality of care to all of our patients. For more information, please visit https://www.pathways-rehab.com
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOCTM), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the hospital-wide conversion ensures that all Pathways Nursing & Rehabilitation Center patients will be cared for using the most technologically and clinically-advanced noninvasive patient monitoring solutions available; risks related to our belief that Masimo SET provides real-time results for all patients to help clinicians to more rapidly assess, diagnose, and treat every patient; risks related to our assumptions regarding the repeatability of clinical results; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at https://www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.

![]()

OU Medical Center Upgrades to Masimo SET® Pulse Oximetry for Improved Patient Outcomes
Oklahoma City & Irvine, California – August 6, 2013 – Masimo (NASDAQ: MASI) today announced that OU Medical Center – Oklahoma's only Level One trauma center for adults and pediatrics, and the state's largest and most comprehensive hospital – has upgraded system-wide to Masimo SET® pulse oximetry, the standard-of-care at leading hospitals around the world.
OU Medical Center, the heart of a 776-bed system that includes The Children's Hospital at OU Medical Center, and OU Medical Center Edmond, joins a growing and distinguished roster of health organizations using Masimo SET® pulse oximetry, clinically shown to virtually eliminate false alarms1 and help clinicians detect life-threatening events.2 OU Medical Center's standardization to Masimo SET® pulse oximetry is in keeping with the healthcare organization's dedication to excellence in care and delivering the best possible results for its patients.
"Since we've been using Masimo pulse oximeters in the NICU, we've seen quite a difference," said Susan Bedwell, clinical nurse specialist, NICU, OU Medical Center. "We don't have as many motion-artifact alarms going off, where we used to have them going off all the time. And we're getting good low perfusion readings, even on our heart-lung bypass patients."
The performance of Masimo SET® pulse oximetry is proven by more than 100 independent and objective studies and thousands of clinical evaluations. Masimo SET® is trusted by clinicians to safely monitor more than 100 million patients each year and is used hospital-wide by eight of the top 10 hospitals on the U.S. News & World Report Best Hospitals Honor Roll (2013-2014). Compared to other pulse oximeters during patient motion and low perfusion, Masimo SET® provides measurements when other pulse oximeters cannot, dramatically reduces false alarms (specificity), and accurately detects true alarms (sensitivity)1 that can indicate a deteriorating patient. Most important, Masimo SET® pulse oximetry has been shown to improve patient outcomes by helping clinicians reduce retinopathy of prematurity (ROP)3 in neonates, screen newborns for critical congenital heart disease (CCHD),4,5 reduce ventilator weaning time and arterial blood gas measurements in the ICU,6 and save lives and costs while reducing rapid response activations and intensive care unit transfers on the general floor.7
"We are honored to partner with OU Medical Center, which has a well-deserved reputation for having patient empathy and providing quality care," said Masimo Founder and CEO Joe Kiani. "We also appreciate and share OU Medical Center's core values – when it comes to doing what's right for patients, the word 'impossible' is not in our respective vocabularies. With this mutual commitment to excellence, we look forward to helping this great hospital and staff, meet and exceed their patient-care needs."
1 Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers".J Clin Anesth. 2012 Aug;24(5):385-91.
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Impact-of-Pulse-Oximetry-Surveillance-on-Rescue.
3 Castillo A, et al. Acta Paediatr. 2011 Feb.;100(2):188-92.
4 de-Wahl Granelli A., et al. BMJ. 2009 Jan 8;338.
5 Ewer A, et al. Health Technol Assess. 2012;16(2):1-184.
6 Durbin, et al. Critical Care Medicine. 2002 Aug.;30(8): 1735 to 1740.
7 Taenzer A, et al. Anesthesiology, 2010 Feb;112(2):282-7.
About OU Medical Center
OU Medical Center's dedication to patient care has remained strong for more than a century and we are positioned to grow even stronger in our second century. As part of OU Medicine, we are always working to provide for the health care of all Oklahomans and to solve medicine's toughest challenges. OU Medical Center, along with The Children's Hospital and OU Medical Center Edmond is Oklahoma's largest and most comprehensive hospital. The downtown campus is located in the heart of Oklahoma City, just minutes from downtown and the beautiful new Bricktown area and the State Capitol. We provide a full range of hospital services for every patient, from the smallest neonate to the most critically ill senior. Visit https://www.OUMedicine.com..
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOCTM), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the hospital-wide conversion ensures that all OU Medical Center patients will be cared for using Masimo SET; risks related to our belief that Masimo SET provides real-time results for all patients to help clinicians to more rapidly assess, diagnose, and treat every patient; risks related to our assumptions regarding the repeatability of clinical results; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at https://www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Scott Coppenbarger
OU Medical Center
Phone: (405) 271-7900 x4
Email: scott.coppenbarger@hcahealthcare.com
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


Masimo Reports Second Quarter 2013 Financial Results
Q2 2013 Highlights (compared to Q2 2012):
- Total revenue, including royalties, rose 12% to $137.4 million
- Product revenue rose 12% to $129.6 million
- Revenue from rainbow® products rose 19% to $11.5 million
- SET® and rainbow® SET unit shipments rose 14% to 42,600
- Net income was $17.0 million, with EPS of $0.30 versus $0.30 in the year-ago period
Irvine, California, July 31, 2013 – Masimo (NASDAQ: MASI) today announced its financial results for the second quarter ended June 29, 2013.
Masimo's total second quarter revenue, including royalties, rose 12% to $137.4 million, compared to $122.8 million for the second quarter of 2012. Second quarter 2013 product revenue rose 12% to $129.6 million, compared to $115.3 million for the second quarter of 2012. The company's worldwide direct product revenue grew 10% in the second quarter of 2013 and represented 83% of product revenue. OEM sales, which accounted for 17% of product revenue, rose 27% compared to the same period in 2012. Revenue from sales of Masimo rainbow products rose 19% to $11.5 million in the second quarter, compared to $9.7 million in the year-ago period. Second quarter 2013 rainbow revenue included $3.6 million in sales of total hemoglobin products.
Net income for the second quarter of 2013 was $17.0 million, or $0.30 per diluted share, compared to net income of $17.7 million, or $0.30 per diluted share, in the second quarter of 2012.
During the second quarter of 2013, the company shipped approximately 42,600 SET pulse oximetry and rainbow SET Pulse CO-Oximetry units, excluding handheld units, up 14% compared to approximately 37,300 in the same prior-year period. Masimo estimates its worldwide installed base as of June 29, 2013 to be 1,148,000 units, up 11% from 1,033,000 units as of June 30, 2012.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "Our financial performance was strong due to the increase in demand for our breakthrough technologies that have been shown to improve care, reduce cost and increase patient safety. The strong second quarter shipments are indicative of the demand for our innovations."
As of June 29, 2013, Masimo's cash and cash equivalents were $78.1 million, compared to $71.6 million as of December 29, 2012. The change reflects primarily net cash generated from operations, offset by $19.8 million in cash used to repurchase one million shares of Masimo common stock in the first half of 2013.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 19905435. After the live webcast, the call will be available on Masimo's website through August 31, 2013. In addition, a telephonic replay of the call will be available through August 14, 2013. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (404) 537-3406 for international callers. Please use reservation code 19905435.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOCTM), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. And in 2012, Masimo acquired the assets of Spire Semiconductor, LLC, a maker of advanced light emitting diode (LED) and other advanced component-level technologies; and PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies and reduce the cost of care; and demand for our technologies. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the integration of acquisitions; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
# # #
Investor Contact: Eli Kammerman
(949) 297-7077
ekammerman@masimo.com
Media Contact: Mike Drummond
(949) 297-7434
mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.
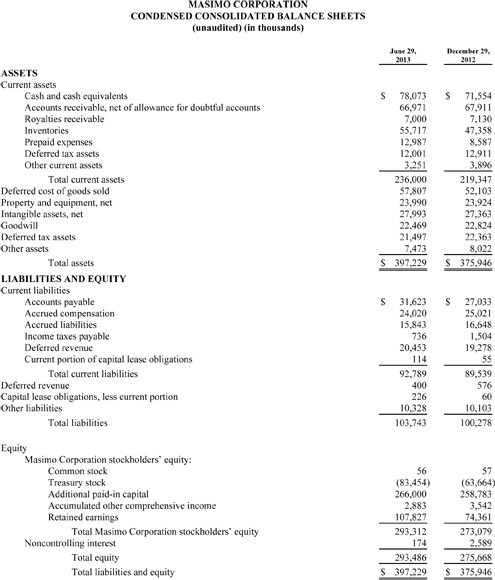
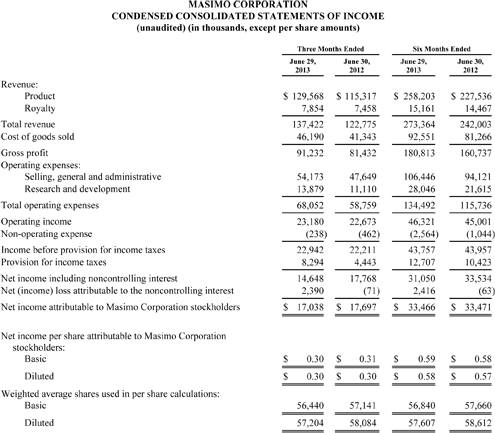
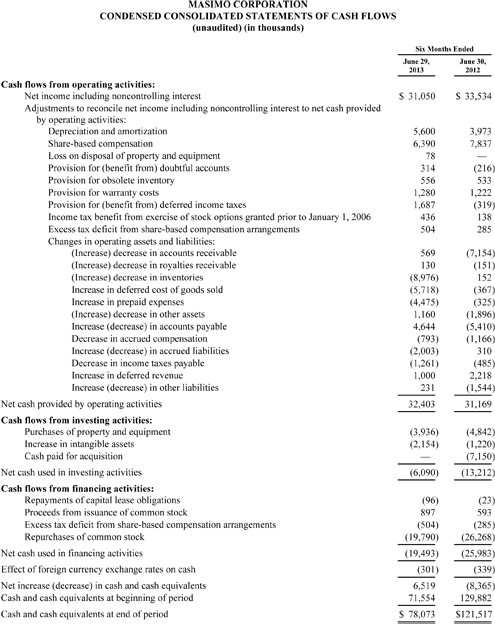


Masimo Announces Eight of Top 10 U.S. Hospitals Now Use Masimo SET® Pulse Oximetry Technology Hospital-Wide
Irvine, California – July 31, 2013 – Masimo (NASDAQ: MASI) today announced that Masimo SET® pulse oximetry is now being used hospital-wide in eight of the top10 hospitals on the current U.S. News & World Report Best Hospitals Honor Roll (2013-2014).
Among the hospitals on the list: Brigham and Women's Hospital, Cleveland Clinic, The Johns Hopkins Hospital, Massachusetts General Hospital, Mayo Clinic, UCLA Medical Center, and UCSF Medical Center.
Masimo SET® pulse oximetry has been shown to virtually eliminate false alarms1 and increase a clinician's ability to detect life-threatening events2 - helping to substantially contribute to improved patient outcomes and patient safety. The clinical accuracy of Masimo SET® pulse oximetry has also been shown to help clinicians significantly reduce retinopathy of prematurity3-5, screen for critical congenital heart disease in newborns6-8, reduce oxygen overdose9, 10 and medical errors11, 12, and save lives in post-surgical floors, recovery2, labor and delivery rooms13, and ICUs14.
More than 100 independent clinical studies have confirmed that Masimo SET® technology allows clinicians to accurately monitor blood oxygen saturation in the most challenging conditions, including patient movement and low peripheral perfusion.15
"We are proud to call these great institutions, our customers; and, we will continue to do our best to serve not only these world-renowned clinical centers, but all of our caring clinical customers, so that they can serve their patients the best way possible," said Masimo Founder and CEO, Joe Kiani.
# # #
1 Shah N, Ragaswamy H, Govindugari K, Estanol L. "Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers." Journal of Clinical Anesthesia. 2012 (10.1016/j.jclinane.2011.10.012)https://www.sciencedirect.com
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Impact-of-Pulse-Oximetry-Surveillance-on-Rescue.
3. Chow L.C., Wright K.W., Sola A.; CSMC Oxygen Administration Study Group. "Can Changes in Clinical Practice Decrease the Incidence of Severe Retinopathy of Prematurity in Very Low Birth Weight Infants?" Pediatrics. 2003 Feb;111(2):339-45.
4. Clinical Practice and SpO2 Technology in the Prevention of ROP in VLBW Infants. Castillo AR, Deulofeut R, Sola A. Presented at Pediatric Academic Societies Annual Meeting May 5-8, 2007.
5. Oxygen as a neonatal health hazard: call for détente in clinical practice. Sola A., Rogido, Marta, Deulofeut, Richard. Acta Paediatrica 2007; 96:801-812.
6. de Wahl Granelli A, Wennergren M, Sandberg K, et al. Impact of pulse oximetry screening on detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ 2009; 338: a3037.
7. Noninvasive Peripheral Perfusion Index as a Possible Tool for Screening for Critical Left Heart Obstruction. Granelli, AD, Ostman-Smith, I. Acta Paediatrica 2007; 96:1455-1459.
8. Screening for Duct-Dependent Congenital Heart Disease with Pulse Oximetry: A Critical Evaluation of Strategies to Maximize Sensitivity. Granelli AD, Mellander M, Sunnegardh J,Sandberg K, Ostman-Smith I. Acta Paediatr 2005; 94:1590-6.
9. Avoiding Hyperoxemia During Neonatal Resuscitation: Time to Response to Different SpO2 Monitors. Baquero H, Alviz R, Sola A. Presented at Pediatric Academic Societies Annual Meeting May 5-8, 2007. A
10. More Reliable Oximetry Reduces the Frequency of Arterial Blood Gas Analyses and Hastens Oxygen Weaning after Cardiac Surgery. Durbin CG, Rostow SK. Crit Care Med 2002; 30(8): 1735-1740
11. Advantages of New Technology Pulse Oximetry with Adults in Extremis. Durbin CG, Rostow SK. Anesth Analg 2002; 94: S81-S83
12. Pulse Oximetry Performance Can Affect Caregiver Time Utilization. Durbin CG, Rostow SK. Anesthesiology 2000;93(3A): A556.
13 Baquero H, Alviz R, Castillo A, Neira F, Sola A. "Avoiding Hyperoxemia During Neonatal Resuscitation: Time To Response Of Different SpO2 Monitors." Acta Paed April 2011 Vol. 100, Issue 4, pp 515-518. Published online https://onlinelibrary.wiley.com/doi/full/10.1111/j.1651-2227.2010.02097.x
14 Goldstein MR, Martin GI, Sindel BD, Furman GI, Ochikubo C, Yand L. SatSeconds Alarm Management Misses Short Desaturations Common to Periodic Breathing and Infantile Apnea. Pediatric Research 2001;49(4):400A/2296.
15 Full Citation List of All Available Clinical Studies Featuring Masimo: http://www.masimo.com/cpub/clinical-masimo-set.htm
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOCTM), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo SET provides real-time results for all patients to help clinicians to more rapidly assess, diagnose, and treat every patient; risks related to our assumptions regarding the repeatability of clinical results; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Community Health Ventures Signs New Supplier Agreement with Masimo
Contract Includes Pronto-7® and Pronto®
Irvine, California – July 23, 2013 – Masimo (NASDAQ: MASI) announced today that it has entered a new year-to-year group purchasing agreement with Community Health Ventures (CHV), the business development affiliate of the National Association of Community Health Centers (NACHC), representing more than 9,000 health centers nationwide. The new agreement allows CHV participants, at their discretion, to take advantage of special pricing and terms pre-negotiated by CHV for Masimo's Pronto-7® and Pronto®.
 The palm-sized Masimo Pronto-7 offers noninvasive SpO2, hemoglobin, pulse rate, and PI spot-check results. |
The Pronto-7 and Pronto offer noninvasive and spot-check testing of total hemoglobin (SpHb), as well as SpO2, pulse rate (PR), and perfusion index (PI).
Both the Pronto-7 and Pronto will be available to CHV's U.S. customer base of community health centers, which serve the primary health care needs of more than 22 million people.
"We are extremely pleased to be partnered with Masimo, a leading developer of innovative technology to improve patient care and reduce cost of care. We're particularly excited to make health centers aware of Masimo's Pronto-7 and Pronto devices," said Gwen Siebert, Community Health Ventures Chief Operating Officer. "Health centers are recognized leaders in the provision of high quality primary care. This is due in large part to health centers using cutting-edge technology to improve their practice and standard of care. We're happy to promote Masimo products through the National Association of Community Health Center's (NACHC) endorsed program, the Value in Purchasing (ViP) program, and look forward to introducing health centers to these exciting, noninvasive innovations in patient care."
Jon Coleman, President, Worldwide Sales, Professional Services and Medical Affairs, stated, "We are happy to partner with Community Health Ventures to support their health centers in advancing care for their patients with noninvasive spot-checking of total hemoglobin, SpO2, pulse rate, and perfusion index."
About Community Health Ventures
Community Health Ventures (CHV) is the business development affiliate of the National Association of Community Health Centers (NACHC), organized in 1971. Founded in 2000, CHV was created under the direction of health center leadership and tasked with creating workable solutions to the tremendous economic pressures facing today's health centers. By negotiating group-purchasing agreements, staffing solutions, lab agreements and more, CHV helps health centers reduce costs and remain competitive. CHV's dedicated staff and management have over 50 years of combined Community Health Center experience and ensure that our programs meet the specialized needs of health centers. For more information, please visit https://www.communityhealthventures.com.
About the National Association of Community Health Centers (NACHC)
Founded in 1970, the National Association of Community Health Centers (NACHC) is a non-profit organization whose mission is to enhance and expand access to quality, community-responsive health care for America's medically underserved and uninsured. NACHC represents the nation's network of over 1,200 Federally Qualified Health Centers (FQHCs), which serve over 20 million people through over 8,000 sites located in all of the 50 states, the District of Columbia, Puerto Rico, the U.S. Virgin Islands, and Guam. For more information, please visit https://www.nachc.org
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to the factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at https://www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo to Report Second Quarter 2013 Financial Results after Market Close on Wednesday, July 31
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., July16, 2013 -- Masimo (NASDAQ: MASI) announced today that it will release second quarter 2013 financial results for the period ended June 29, 2013, after the market closes on Wednesday, July 31, 2013. The conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at https://www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 19905435. After the live webcast, the call will be available on Masimo's website through August 31, 2013. In addition, a telephonic replay of the call will be available through August 14, 2013. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 19905435.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. And in 2012, Masimo acquired the assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET®and Masimo rainbow®SET®technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Contact:
Eli Kammerman
(949) 297-7077
ekammerman@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo Unveils EMMA™ Mainstream Capnograph for Enhanced Assessment of End-Tidal Carbon Dioxide
Irvine, California – July 15, 2013 – Masimo (NASDAQ: MASI), inventor of breakthrough Measure-Through Motion and Low Perfusion pulse oximetry, today announced the release of EMMA™ Capnograph with waveform display, offering clinicians greater assessment of end-tidal carbon dioxide (EtCO2) and respiration rate, as well as assisting in recognition of return to spontaneous circulation, for a variety of clinical settings, including emergency medicine and transport, ORs, ICUs, patient rooms, and clinics.
 Masimo EMMA™ Capnograph. |
Rugged, water-resistant and operational in first-responder and other clinically challenging conditions, EMMA Capnograph displays and monitors respiratory rate and EtCO2 continuously with full accuracy within 15 seconds when connected to a patient's breathing circuit. Powered by two standard AAA batteries, EMMA's portability allows for easy use during cardiopulmonary resuscitation (CPR) and intubation in multiple points of care.
EMMA's proprietary hydrophilic coating prevents water droplets from optically interfering with the device's internal infrared beam that helps detect EtCO2. This obviates the need for heaters, while enabling faster start-up with no warm-up period and far less power consumption. And because EMMA is integrated into the breathing circuit for easy viewing during CPR and endotracheal tube placement, it is highly accessible during transport and/or emergency ventilation scenarios – allowing quick assessment in just seconds for adult, pediatric, and infant patients.
"Masimo's EMMA capnograph with this improved continuous waveform display gives immediate and continuous confirmation of endotracheal tube placement, even during cardiac arrest, making it compliant with AHA/ILCOR recommendations," said Dr. Daniel Davis, Professor of Clinical Emergency Medicine, and Director of the UCSD Center for Resuscitation Science in San Diego, California. "In addition, EMMA can guide ventilation in critically ill and injured patients and allows assessment of the adequacy of cardiopulmonary resuscitation and identification of return of spontaneous circulation. The small size also makes it ideal for critical resuscitations by allowing waveform capnography to be brought to the patient."
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to the performance of EMMA and its ability to provide end-tidal CO2 assessment during cardiopulmonary resuscitation (CPR) and intubation for all patients in all clinical settings in just a few seconds; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


French Society of Anaesthesia and Intensive Care Adds Masimo PVI® to Guidelines for Perioperative Hemodynamic Optimization
Irvine, California – July 11, 2013 – Masimo (NASDAQ: MASI) announced today that the French Society for Anaesthesia and Intensive Care (SFAR) has added Masimo PVI® – a noninvasive and continuous parameter to help clinicians assess fluid responsiveness and improve fluid management – to guidelines for optimal hemodynamic management during surgery.1
During surgery, the optimal administration of fluid has been shown to reduce post-operative mortality, length of hospital stay, and the recovery period of patients who have undergone gastrointestinal surgery. However, SFAR noted some traditional management approaches to guide fluid administration are unreliable, and that central venous pressure (CVP) monitoring – a common invasive parameter – "constitutes a notoriously invasive and insufficient approach." SFAR added, "Particularly in high risk surgery, the classic monitoring of hemodynamic variables does not ensure adequate systemic perfusion (risk of occult 'hypovolemia') and may lead to inadequate treatment decisions."
Instead of so-called "static" parameters such as CVP, SFAR recommends the use of "dynamic" parameters that measure variations over the respiratory cycle. Multiple dynamic parameters have been shown to help clinicians predict fluid responsiveness and improve fluid management. However, most dynamic parameters require invasive and/or complex methods. In contrast, PVI is noninvasive and easily obtained with any Masimo SET® or rainbow® sensor. In recommending the use of dynamic parameters, SFAR noted, "PVI, whose reliability has been proven,2,3 is available today, and could allow the spread of preload dependence monitoring for patients under general anaesthesia and controlled ventilation in minor and moderate surgery."
SFAR said dynamic indices such as PVI contribute "to the detection of the 'low probability of response to filling' as long as the value remains low (<9%), eliminating the need to test the response to filling in a regular systemic manner," and that compared to traditional "empirical administration of fluids," which can either be insufficient or excessive, a dynamic indicator such as PVI "allows an improvement in the patient's prognosis."
SFAR also observed that, "If the main effect of the vascular filling (VF) is to increase venous return and thus cardiac output, it is surprising that cardiac output is only rarely monitored in current practice by anaesthetists." In alluding to PVI, SFAR stated: "Only rapid implementation, surgeon-independent strategies which are either noninvasive or minimally invasive and of low cost will be able to spread in practice."
The new SFAR Formalized Experts' Recommendations add to the growing catalog of clinical evidence in support of Masimo's breakthrough, noninvasive and continuous monitoring technologies, such as PVI. Just last year, the United Kingdom's National Health Service included PVI in its Intra Operative Fluid Management pack, which serves as a guide for hospitals wishing to implement fluid responsiveness monitoring to improve patient outcomes.4
"In our randomized clinical trial,5 we found that Masimo PVI-based goal-directed fluid management helps to improve perioperative vascular filling, saving unnecessary fluids and reducing intraoperative and postoperative lactate levels," said Patrice Forget, MD, anesthesiologist, Department of Anesthesiology at St-Luc University Hospital in Brussels, Belgium.
Joe Kiani, Founder and CEO of Masimo, stated: "We are delighted that PVI has proven so useful and that leading international medical bodies such as SFAR and NHS offer powerful validations of PVI. Our mission has been and continues to be to 'improve patient outcome and reduce cost of care by taking noninvasive monitoring to new sites and applications.' PVI has certainly been shown to do just that. We are honored that some of the world's leading medical authorities are making PVI the standard of care."
1 Vallet B., Blanloeil Y., Cholley B., Orliaguet G., Pierre S., Tavernier B. "Strategy for perioperative vascular filling - Guidelines for perioperative haemodynamic optimization." Experts' Formalized Recommendations, French Society of Anaesthesia and Intensive Care (SFAR), Validation by the administrative council of SFAR on 19 October 2012. Available https://pubmed.ncbi.nlm.nih.gov/24126197/.
2 Cannesson M, Desebbe O, Rosamel P, Delannoy B, Robin J, Bastien O, Lehot JJ. Pleth variability index to monitor the respiratory variations in the pulse oximeter plethysmographic waveform amplitude and predict fluid responsiveness in the operating theatre. Br J Anaesth 2008;101:200-6
3 Zimmermann M, Feibicke T, Keyl C, Prasser C, Moritz S, Graf BM, Wiesenack C. Accuracy of stroke volume variation compared with pleth variability index to predict fluid responsiveness in mechanically ventilated patients undergoing major surgery. Eur J Anaesthesiol 2010;27:555-61
4 http://www.prweb.com/releases/2013/3/prweb10476496.htm
5. Forget P, Lois F, de Kock M. Goal-Directed Fluid Management Based on the Pulse Oximeter-Derived Pleth Variability Index Reduces Lactate Levels and Improves Fluid Management. Anesth Analg 2010;111(4):910-4
*To see a summary of all known clinical studies and abstracts on Masimo technologies and noninvasive measurements, please visit: http://www.masimo.com/cpub/clinicals.htm.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including PVI, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


The Joint Commission Issues National Patient Safety Goal and Requirements for Medical Device Alarm Management
Masimo SET® Measure-Through Motion and Low Perfusion Pulse Oximetry and Masimo Patient SafetyNet™ Shown to Help Clinicians Reduce False Alarms and Improve Patient Outcomes
Irvine, California – July 8, 2013 – Masimo (NASDAQ: MASI) announced today that the Joint Commission has issued a National Patient Safety Goal on Alarm Management to improve the safety of clinical alarm systems, requiring hospital leaders to establish alarm safety as a hospital priority and take specific actions to help avoid patient injuries and deaths related to alarms. The National Patient Safety Goal follows the Joint Commission's recent Sentinel Event Alert on medical device alarm safety.
The Joint Commission recognizes that while clinical alarms are intended to alert caregivers to potential patient problems, if alarms are not properly managed they can compromise patient safety. According to the Joint Commission, the most common cause of alarm-related sentinel events was alarm fatigue. Alarm fatigue happens when monitoring technologies produce false alarms.
Pulse oximeters are the most common form of monitoring in most hospitals, but up to 77% of alarms with conventional pulse oximeters can be false, due to patient movement and/or low perfusion.1 False alarms created by inaccurate measurements from motion artifact or low perfusion can increase nursing workload and alarm fatigue, which can lead to a delayed response, compromised care, and poor patient outcomes.2
In 1996, Masimo launched Measure-Through Motion and Low Perfusion Pulse Oximetry, called SET® or Signal Extraction Technology®. Masimo SET® pulse oximetry has been shown in clinical studies to dramatically reduce false alarms3 and increases a clinician's ability to detect life-threatening events4 – helping to substantially contribute to improved patient outcomes and patient safety. A side-by-side comparison published last year in the Journal of Clinical Anesthesia showed Masimo SET® pulse oximetry had higher oxygen saturation (SpO2) specificity and sensitivity and lower failure rates during conditions of motion and low perfusion than the Nellcor N-600 (OxiMax V 1.1.2.0) and the GE TruSat (formerly Datex-Ohmeda).3
| Masimo SET®(v5.0) | Nellcor N-600 (v1.1.20) | GE TruSat | |
|---|---|---|---|
|
Missed True Alarms |
3% |
43% |
83% |
|
False Alarms |
5% |
28% |
18% |
The ability of Masimo SET® to accurately detect oxygen saturation changes (sensitivity) and avoid false alarms (specificity) has been validated in more than 100 independent and objective studies,3 internal studies, and thousands of clinical evaluations. Most important, only Masimo SET® pulse oximetry has been shown to improve patient outcomes by helping clinicians reduce retinopathy of prematurity, screen newborns for critical congenital heart disease,5,6 reduce ventilator weaning time,7 and save lives while reducing rapid response activations and intensive care unit transfers on the general floor.4
Hospitals can further improve patient outcomes and comply with the Joint Commission recommendations by using Masimo Patient SafetyNet™, which combines the performance of Masimo SET® pulse oximetry, the enabler of reliable monitoring in the general ward, with ventilation monitoring and wireless clinician notification. Patient SafetyNet can help ensure patients' safety by noninvasively and continuously measuring and tracking their underlying physiological conditions and changes that signal declining health status in real-time. When changes occur in the measured values, which may indicate deterioration in the patient's condition, the system automatically sends wireless alerts directly to clinicians – prompting a potentially lifesaving response to the patient's bedside. Patient SafetyNet has been clinically shown to reduce preventable and costly rescue events, transfers to intensive care units, and deaths related to opioid-induced respiratory depression.8
The Joint Commission's National Patient Safety Goal of improving clinical alarm systems will be effective in two phases in 2014 and 2016:
- As of July 1, 2014, hospital leaders establish alarm safety as a hospital priority
- During 2014, hospitals identify the most important alarm signals to manage
As of January 1, 2016, hospitals should establish policies and procedures for managing the alarms identified that, at a minimum, address:
- Clinically appropriate settings for alarm signals
- When alarm signals can be disabled
- When alarm parameters can be changed
- Who in the organization has the authority to set alarm parameters
- Who in the organization has the authority to change alarm parameters
- Who in the organization has the authority to set alarm parameters to "off"
- Monitoring and responding to alarm signals
- Checking individual alarm signals for accurate settings, proper operation, and detectability
Masimo's Radical-7® and Rad-87® Pulse CO-Oximeters have a unique Alarm Status Indicator that can notify clinicians if the alarm settings have been changed from the hospital selected default. And a Device Profile Indicator verifies through color-coding that the devices are in the correct care areas (i.e., neonatal or adult) and that the alarms are configured properly.
Joe Kiani, Founder & CEO of Masimo, stated: "We applaud the Joint Commission for focusing attention on problems with medical device alarms, which consistently rank among the top most critical health technology hazards in hospitals. We launched Masimo nearly 25 years ago with a mission to eradicate pulse oximetry false alarms – one of the most frequent false alarms plaguing hospitals. Clinicians have told us that countless patients have tragically died due to alarm fatigue, and that the performance of Masimo SET® Pulse Oximetry is a major reason they have embraced our technology."
Dr. Michael O'Reilly, Chief Medical Officer for Masimo, stated: "By solving the pulse oximeter's motion artifact limitation with Signal Extraction Technology®, Masimo has made a huge contribution to patient care and helped push safety monitoring to areas where patients previously weren't monitored due to the significant false-alarm rate associated with conventional pulse oximetry."
1 Wiklund L, Hok B, Stahl K, Jordeby-Jonsson A. Postanesthesia monitoring revisited: incidence of true and false alarms from different monitoring devices. J Clin Anesth 1994;67:182–8.
2 Cvach M. Monitor Alarm Fatigue, An Integrative Review. Biomedical Instrumentation & Technology. July/August 2012.
3 Shah N, Ragaswamy H, Govindugari K, Estanol L. "Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers." Journal of Clinical Anesthesia. 2012 (10.1016/j.jclinane.2011.10.012) https://www.sciencedirect.com
4 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Impact-of-Pulse-Oximetry-Surveillance-on-Rescue.
5 de Wahl Granelli A, Wennergren M, Sandberg K, et al. Impact of pulse oximetry screening on detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ 2009; 338: a3037.
6 Ewer A, et al. Health Technol Assess. 2012;16(2):1-184.
7 Durbin, et al. Critical Care Medicine. 2002 Aug.;30(8): 1735 to 1740.
8 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The DartmouthExperience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. Available online.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms all other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, the company introduced Masimo Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET® and Masimo rainbow SET® technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo Patient SafetyNet can help keep patients safer by noninvasively, continuously measuring and tracking their underlying physiological condition to help hospitals avoid preventable patient deaths and injuries associated with failure to rescue events, risks related to our assumptions of the repeatability of clinical results obtained, and risks related to the system's ability to significantly decrease traumatic critical events and costly ICU transfers to help improve patient outcomes and reduce costs; risks related to our belief that Masimo SET virtually eliminates false alarms and increases a clinician's ability to detect life-threatening events; risks related to our belief that Adaptive Threshold Alarm helps reduce nuisance alarms by automatically adjusting the audible alarm to the patient's baseline; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at https://www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.



South Shore Hospital Standardizes to Masimo SET® Pulse Oximetry for Improved Patient Monitoring
Weymouth, Massachusetts & Irvine, California – June 19, 2012 – Masimo (NASDAQ: MASI) and South Shore Hospital, ranked among the top three hospitals in Massachusetts by U.S. News & World Report (2012-13), jointly announce that South Shore Hospital has converted to Masimo SET® pulse oximetry and sensor technologies, used at leading health organizations worldwide.
Masimo SET® pulse oximetry virtually eliminates false alarms1 and increases a clinician's ability to detect life-threatening events2 – helping to substantially contribute to improved patient outcomes and patient safety. Masimo SET® pulse oximetry also has been shown to help clinicians reduce retinopathy of prematurity3, detect critical congenital heart disease in newborns4, and save lives in post-surgical floors, recovery2, labor and delivery rooms5, and ICUs6.
"With Masimo SET®, we're able to get better SpO2 measurements in patients during states of low perfusion and motion artifact," said Charlie Arienti, Director of Respiratory Therapy at South Shore Hospital. "With Masimo, the signal processing is better and the results are reliable."
South Shore Hospital's conversion to Masimo SET® pulse oximetry is in keeping with the healthcare organization's dedication to excellence in care and delivering the best possible results for its patients. More than 100 independent clinical studies have confirmed that Masimo SET® technology allows clinicians to accurately monitor blood oxygen saturation in the most challenging conditions, including patient movement and low peripheral perfusion.
"We are happy to have forged this great partnership with South Shore Hospital, which like other leading healthcare organizations is demonstrating its commitment to patient safety and care by taking advantage of our breakthrough Measure-Through Motion and Low Perfusion pulse oximetry technology performance," said Masimo Founder and CEO Joe Kiani. "We value this relationship with South Shore, and together look forward to helping their skilled and dedicated staff meet and exceed their patient care needs."
1 Shah N, Ragaswamy H, Govindugari K, Estanol L. "Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers." Journal of Clinical Anesthesia. 2012 (10.1016/j.jclinane.2011.10.012) https://www.sciencedirect.com
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Impact-of-Pulse-Oximetry-Surveillance-on-Rescue.
3 Chow L.C., Wright K.W., Sola A.; CSMC Oxygen Administration Study Group. "Can Changes in Clinical Practice Decrease the Incidence of Severe Retinopathy of Prematurity in Very Low Birth Weight Infants?" Pediatrics. 2003 Feb;111(2):339-45.
4 de Wahl Granelli A, Wennergren M, Sandberg K, et al. Impact of pulse oximetry screening on detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ 2009; 338: a3037.
5 Baquero H, Alviz R, Castillo A, Neira F, Sola A. "Avoiding Hyperoxemia During Neonatal Resuscitation: Time To Response Of Different SpO2 Monitors." Acta Paed April 2011 Vol. 100, Issue 4, pp 515-518. Published online here
6 Goldstein MR, Martin GI, Sindel BD, Furman GI, Ochikubo C, Yand L. SatSecondhttp://trk.cp20.com/Tracking/t.c?63r7p-awv9b-me8qtn0&_v=2arm Management Misses Short Desaturations Common to Periodic Breathing and Infantile Apnea. Pediatric Research 2001;49(4):400A/2296.
About South Shore Hospital
South Shore Hospital is a 378-bed, not-for-profit, tax-exempt, charitable provider of acute, emergency, outpatient, home health, and hospice care to the people of Southeastern Massachusetts. South Shore Hospital's home care division includes South Shore Visiting Nurse Association, Hospice of the South Shore, and Home & Health Resources. The hospital's 900-member medical staff represents all leading medical specialties. South Shore Hospital employs 3,800 people, supported by a team of 600 volunteers. South Shore Hospital is licensed to provide Level II Trauma care and Level III Maternal/Newborn care. South Shore Hospital has been ranked among the top three hospitals in Massachusetts, according to the 2012 U.S. News & World Report Best Hospitals report and named a Top Place to Work by The Boston Globe. Please call (781) 624-6673 or visit southshorehospital.org for a referral to a South Shore Hospital physician.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the hospital-wide conversion ensures that all South Shore Hospital patients will be cared for using the most technologically and clinically-advanced noninvasive patient monitoring solutions available; risks related to our belief that Masimo SET provides real-time results for all patients to help clinicians to more rapidly assess, diagnose, and treat every patient; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at https://www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Sarah Darcy
South Shore Hospital
Phone: (781) 624-8970
Email: sarah_darcy@sshosp.org
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.



Masimo Signs Far-Reaching Pulse Oximetry Distribution Deal with Butler Schein Animal Health
Agreement with Leading Supplier Advances National Push into the Animal Healthcare Market
Irvine, California – June 11, 2013 – Masimo (NASDAQ: MASI) announced today that Butler Schein Animal Health, the U.S. animal health business of Henry Schein, Inc. (NASDAQ: HSIC), is now a distributor of Masimo's breakthrough line of pulse oximetry technologies.
Surgical anesthesia used on animals can severely limit peripheral perfusion or blood circulation to the extremities, sometimes making it nearly impossible to get accurate oxygenation and pulse rate readings without the proper technology. Inaccurate readings can delay proper diagnosis and treatment, potentially putting the animal patient's life at risk. Post-surgery, animal patients also need to be monitored for respiratory and cardiovascular problems – a major cause of post-operative death among dogs and cats.1
Masimo's upgradable rainbow® Radical-7® Pulse CO-Oximeter, Rad-87®, Rad-57®, Rad-5® and Rad-8® – all with SET® Measure-Through Motion and Low Perfusion pulse oximetry – will be available to Butler Schein's U.S. client base of more than 26,000 veterinary professionals in all 50 states.
Masimo's technology is designed to provide clinicians oxygen saturation (SpO2) measurements under the most challenging conditions – patient motion and low perfusion, which are common in animal healthcare. Masimo SET® virtually eliminates false alarms2 and increases a clinician's ability to detect life-threatening events.3
"Masimo SET® technology has shown that pulse oximetry can be used reliably in field procedures as well as in the hospital setting," said Lin Klein, VMD, DACVA, who specializes in providing anesthesia services for tigers, leopards, cheetahs, and lions in zoos. "SET® has all but eliminated perfusion-related issues as a source of worry and frustration due to failed or inaccurate SpO2 readings."
"We are thrilled that Butler Schein Animal Health has added Masimo pulse oximetry technology to its impressive line-up of products," said Jon Coleman, President of Worldwide Sales and Marketing and Clinical Research. "Animal healthcare is an important and growing field, and offers Masimo yet another opportunity to take our noninvasive monitoring to new sites and applications."
1 Brodbelt D, Blissitt K, Hammond R, Neath P, Young L, Pfeiffer D, Wood J. "The risk of death: the Confidential Enquiry into Perioperative Small Animal Fatalities." Veterinary Anaesthesia and Analgesia, 2008, 35, 365–373
2 Shah N, Ragaswamy H, Govindugari K, Estanol L. "Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers." Journal of Clinical Anesthesia. 2012 (10.1016/j.jclinane.2011.10.012) https://www.sciencedirect.com
3 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Impact-of-Pulse-Oximetry-Surveillance-on-Rescue.
About Butler Schein Animal Health
Butler Schein Animal Health (Butler Schein) – the U.S. animal health business of Henry Schein, Inc. is the leading companion animal health distribution company in the United States headquartered in Dublin, Ohio. Butler Schein employs approximately 900 team members including 300 field sales representatives and 200 telesales and customer support representatives. With 15 strategically positioned, state-of-the-art distribution facilities and 10 inside sales centers nationwide, we maintain 99%+ order-fill ratio, accomplishing our mission of providing the right product at the right place and at the right time.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SET delivers more reliable measurements, virtually eliminates false alarms, and increases a clinician's ability to detect life-threatening events, can be used reliably in field procedures as well as in the hospital setting on animals, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at https://www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


University Children's Hospital Basel Installs Masimo Patient SafetyNet™ System for Advanced Care and Oversight of General Ward Patients
Installation is Part of the Hospital-Wide Conversion to Masimo SET®Pulse Oximetry
Basel, Switzerland & Irvine, California – June 6, 2013 – University Children's Hospital Basel in Basel, Switzerland, and Masimo (NASDAQ: MASI) today announced that the hospital has become the first multi-department pediatric facility in Central Europe to install on all general ward beds Masimo Patient SafetyNet™, a remote monitoring and clinician notification system shown to keep patients safer, enabling a 65% reduction in rapid response team activations and 48% reduction in ICU transfers.1
The installation at the University Children's Hospital Basel – Universitäts-Kinderspital Beider Basel (UKBB) – took place after an extensive evaluation process resulting in the organization's standardization to Masimo SET® pulse oximetry.
"We are pleased about the improved monitoring quality for patients on all our general wards, covering 80 beds for inpatient care," Ruth Spalinger, Nurse Manager at UKBB said of Patient SafetyNet. "We have observed that it's especially beneficial for our most vulnerable patients, who potentially suffer from respiratory depression. And, our staff has been extremely satisfied with the system."
UKBB joins a growing list of prominent health systems around the world using Patient SafetyNet, which combines the performance of Masimo SET®pulse oximetry, the enabler of reliable monitoring in the general ward, with ventilation monitoring and wireless clinician notification. Patient SafetyNet can help ensure patients' safety by noninvasively and continuously measuring and tracking their underlying physiological conditions and changes that signal declining health status in real-time. When changes occur in the measured values, which may indicate deterioration in the patient's condition, the system automatically sends wireless alerts directly to clinicians – prompting a potentially lifesaving response to the patient's bedside. Patient SafetyNet has been clinically shown to reduce preventable and costly rescue events, transfers to intensive care units, and deaths related to opioid-induced respiratory depression.1
"University Children's Hospital Basel has made the safety of their patients its first priority," said Joe Kiani, founder and CEO of Masimo. "We are thrilled to be partnering with this highly esteemed and modern healthcare facility to help protect young patients, improve outcomes, and reduce costs."
1 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The DartmouthExperience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. Available online.
About University Children's Hospital Basel
As an independent university pediatric center, the Children's Hospital Basel offers full capabilities to care for seriously ill children. In a unique beautiful environment, services of many different pediatric subspecialties are offered for in-and-out patients. The Children's Hospital Basel is a partner of the University of Basel and responsible for education and research related to the care of the full spectrum of pediatric patients. For more information, visit https://www.ukbb.ch/en/ukbb/the-hospital.html.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that all neonatal patients at University Children's Hospital Basel will be cared for using the most technologically and clinically-advanced noninvasive patient monitoring solutions available; risks related to our belief that Masimo Patient SafetyNet can help keep patients safer by noninvasively, continuously measuring and tracking their underlying physiological condition to help hospitals avoid preventable patient deaths and injuries associated with failure to rescue events, risks related to our assumptions of the repeatability of clinical results obtained, and risks related to the system's ability to significantly decrease traumatic critical events and costly ICU transfers to help improve patient outcomes and reduce costs; risks related to our belief that Masimo SET virtually eliminates false alarms and increases a clinician's ability to detect life-threatening events; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at https://www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Sandra Soland or Martina Beranek
Universitäts-Kinderspital Beider Basel (UKBB)
Phone: +41 61 704 17
Email: info@ukbb.ch
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SedLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.

Masimo Announces Root™
Barcelona, Spain – June 3, 2013 – Masimo (NASDAQ: MASI) today at the European Society of Anesthesiology Meeting announced the CE Mark and international Limited Market Release* of Root™, an intuitive patient monitoring and connectivity platform that is destined to transform patient care from the operating room to the general floor. High-impact innovations in Root include:
- Radical-7®- Masimo's breakthrough rainbow® and SET® measurements from Masimo's Radical-7® handheld monitor that docks into Root, instantly interpretable, with a 10-inch high visibility, intuitive navigation touchscreen display
- MOC-9™- Flexible measurement expansion through Masimo Open Connect™ (MOC-9™) with SedLine® brain function monitoring and Phasein™ capnography MOC-9 modules from Masimo or third-party measurement by other companies to expand the platform's measurements & capabilities
- Iris™- Built-in connectivity gateway through Iris™ for standalone devices such as IV pumps, ventilators, hospital beds, and other patient monitors
- MyView™-Automatic display of parameters, waveforms, and viewing configuration based on the clinician's presence through MyView™ technology
Instantly Interpretable, High Visibility Display of the Radical-7's Breakthrough Measurements
Root provides a docking station for Masimo's Radical-7 handheld monitor to enable instant interpretation of its breakthrough measurements on a high visibility, brilliant 10-inch touchscreen display. The adaptive display provides waveforms along with either a trend view in which each measurement value is displayed alongside a graph of its values over time, or an analog view for quick assessment through gauges showing measurement values in relation to alarm ranges.
When docked with Root, the Radical-7's screen transforms into an alarm status visualizer, with a three-dimensional, anatomical image that associates device measurements with anatomical alarm status.
Root's display helps clinicians fully utilize the Radical-7's capabilities with noninvasive and continuous monitoring of:
- Total hemoglobin (SpHb®) to help clinicians reduce blood transfusions and detect bleeding1,2,3
- Pleth Variability Index (PVI®) to help clinicians assess fluid responsiveness and improve fluid management4,5,6,7
- Acoustic respiration rate (RRa™) for patient-tolerant ventilation monitoring with similar accuracy as capnography8
- Accurate oxygen saturation (SpO2) and pulse rate during challenging conditions with Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry,9 proven to help clinicians reduce retinopathy of prematurity,10 screen newborns for critical congenital heart disease,11,12 reduce ventilator weaning time,13 and save lives while reducing rapid response activations and intensive care unit transfers on the general floor."14
Intuitive, Touchscreen Navigation for Easy and Adaptable Use in Any Environment
With a simple tap, swipe, or drag-and-drop, views, sizing, and settings can be customized on the fly to suit any environment, clinician preference, or patient-specific need. This allows Root to be adaptively used across the continuum of care – even when needs vary widely by care area.
"Root displays rainbow® measurements in a manner so that at any moment I can see what I want to see and how I want to see it – a great advantage to the anesthesiologist in a data-rich operating room," stated Dr. Keith Ruskin, Professor of Anesthesiology at Yale-New Haven Hospital in New Haven, Conn. "Root makes it easy to use SpHb and PVI together to optimize transfusions and perform goal-directed fluid management."
Flexible Measurement Expansion through Masimo Open Connect™ (MOC-9™)
MOC-9, along with the Radical-7's breakthrough measurements, work together to create the ultimate patient monitoring platform for flexible use anywhere in the hospital, from the operating room to the general floor, conscious sedation, and intensive care unit.
The SedLine brain function monitoring MOC-9 module for Root advances brain function monitoring to improve the care of patients under anesthesia or sedation. The core product is a state-of-the-art EEG-based brain function monitor utilizing four simultaneous EEG channels. SedLine measures the effects of anesthesia and sedation by monitoring electrical activity on both sides of the brain at the same time, to enable more individualized titration and improve the care of patients under anesthesia or sedation. With demonstrated reliability under challenging clinical conditions and superior resistance to cautery, SedLine offers a cost-effective solution for brain function monitoring that helps clinicians achieve targeted sedation throughout all phases of anesthesia—in the OR and ICU.
The Phasein capnography MOC-9 module for Root provides precise ETCO2 and respiratory rate measurements with crisp waveforms and virtually no start up time. In addition, with the Nomoline™ "No Moisture" sample line, customers can choose from a single patient use Nomoline version – designed for extended life in high humidity environments – or the multi-use Nomoline adapter for cost-effective, repeated use on different patients with only the cannula itself being replaced with each patient.
"Combining Masimo SET® Pulse Oximetry and the breakthrough noninvasive rainbow® measurements with specialty measurements like brain function monitoring and capnography in the same platform creates a new level of safety and convenience," stated Dr. Michel Struys, Professor and Chair of Anesthesiology at the University of Groningen in the Netherlands. "Instead of using multiple standalone devices, we can now see everything on one screen to streamline care and improve efficiency."
Designed for Third-Party Development of Expanded Measurements
Root is also designed to allow other companies to expand the platform's measurements with their own measurements through MOC-9 by following Masimo's established development and validation process.
Rick Fishel, Masimo's President of Worldwide OEM Business, stated: "As a serial innovator, Masimo understands that disruptive technologies that improve patient care rarely come from large, established companies. Market barriers and development costs often keep small, innovative companies from delivering their products to the clinicians and patients who need them most. With Root, Masimo is providing an open invitation to other companies, from small to large, to develop and commercialize their innovations through Masimo's ever-expanding customer base."
Built-in Connectivity Gateway through Iris™
Despite medical technology advances, the lack of device communication and integration creates risks to patient safety in hospitals around the world. Without device interoperability, critical patient information can go unnoticed – leaving busy clinicians in the dark and vulnerable patients in danger. Existing approaches for device interoperability require separate hardware, software, and/or network infrastructure, which can clutter the patient room, increase complexity, burden IT management, and increase costs.
To address these challenges, each Root can also be used as a connectivity gateway to integrate multiple standalone devices such—as IV pumps, ventilators, hospital beds, and other patient monitors—when used as part of the optional Iris connectivity package in Masimo Patient SafetyNet™. Iris allows standalone device information to be remotely viewed with Patient SafetyNet, transmitted through notification systems or sent to electronic health record (EHR) systems to facilitate better patient care and meaningful use. Device connectivity with Iris is designed to leverage existing network infrastructures and reduce costs while enhancing clinical workflows and decision support to improve patient safety, whether the clinician is at the bedside, down the hall, or across the globe.
MyView Technology
MyView is a wireless, presence detection badge system that enables clinicians to automatically display customized clinical profiles on Masimo devices, such as Root. When a clinician approaches the bedside, a clinician-worn MyView badge signals the Radical-7 and Root to display a pre-selected set of parameters and waveforms tailored to the individual clinician's preferences for easy viewing. Masimo measurements and the flexibility with which they can be displayed continue to expand; however, this doesn't mean that all clinicians need to see all of the information in the same way. While a physician may want to see all parameters and waveforms, a medical assistant may want to see a few parameters and no waveforms.
"Root represents nearly 25 years of relentless innovation aimed at improving patient safety," said Joe Kiani, founder and CEO of Masimo. "With Root, Masimo seeks to usher in a new era of patient care. Root not only enables breakthrough measurements to become ubiquitous, but displays those measurements in an easily interpreted manner alongside integrated data from multiple devices to automate the process of care and improve patient safety. To facilitate the transformation of patient care, Root will be offered at a breakthrough price point, too."
For more information on Root, visit http://www.masimo.co.uk/root.*
Companies interested in developing a MOC-9 measurement can request more information online at: https://www.masimo.co.uk/products/continuous/moc9/.
The Root™ Monitoring and Connectivity Platform is CE Marked and the information contained in this release is applicable to audiences in European CE Mark countries only.
1 Ehrenfeld J.M., et al. American Society of Anesthesiologists. 2010;LB05.
2 Awada W.F.N., et al. Proceeding of the Society for Technology in Anesthesia Annual Meeting, 2013: p. 51.
3 Shander et al. Transfusion. 2010;50(4):753-765.
4 Cannesson M, et al. Br J Anaesth. 2008 Aug;101(2)200-6
5 Loupec T, et al. Crit Care Med. 2011 Feb;39(2):294-9.
6 Byon H, et al. Br J Anaesth. 2013 Apr;110(4):586-91.
7 Forget P, et al. Anesth Analg. 2010 Oct;111(4):910-4.
8 Ramsay M.A.E., et al. Anesth. Analg 2013 June;,ANE.0b013e318290c798.
9 Shah N, et al. J Clinical Anaesthesia. 2012 Aug;24(5):385-91.
10 Castillo A, et al. Acta Paediatr. 2011 Feb.;100(2):188-92.
11 de-Wahl Granelli A., et al. BMJ. 2009 Jan 8;338.
12 Ewer A, et al. Health Technol Assess. 2012;16(2):1-184.
13 Durbin, et al. Critical Care Medicine. 2002 Aug.;30(8): 1735 to 1740.
14 Taenzer A, et al. Anesthesiology, 2010 Feb;112(2):282-7.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our assumptions regarding timing of regulatory clearances and commercial availability in other countries; risks related to our assumptions that Root will provide a connectivity platform for any standalone device; and risks related to our assumption that Root will support/enable any third-party development of new measurements, technologies, product through MOC-9; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium. All other trademarks, service marks, trade names, logos and icons, registered or not, are the property of third parties as named/indicated.
Press Note: The included product photos illustrating various features and display screens on Root™ are available for media use and publication only.


Masimo Renews $1,000,000 Guarantee that Masimo SET® Will Outperform All Covidien Nellcor™ Pulse Oximeters
The Renewal Addresses Recent Covidien Announcements and Potential Confusion Over Pulse Oximetry Performance Crucial to Patient Care
Irvine, California – May 30, 2013 – Masimo (NASDAQ: MASI), the inventor of measure-through motion and low perfusion pulse oximetry today renewed its $1,000,000 (USD) guarantee that Masimo SET® Pulse Oximetry and rainbow® Pulse CO-Oximetry™ will outperform all Nellcor™ pulse oximeters, including the three that Covidien recently announced have received FDA 510(k) clearance with motion claims.1,2 This offer is available only to hospitals whose goal is to upgrade their pulse oximetry hospital-wide to the new standard. Important details, conditions, and qualifications for the guarantee are available at http://www.masimo.com/1millionrules.htm.3
"Masimo has always stood for the truth," stated Joe Kiani, Founder and CEO of Masimo. "Covidien's recent announcements appear to be an attempt to create an impression that Nellcor products are clinically equivalent, or even superior, to Masimo's products. In fact, the performance of Nellcor pulse oximeters has not changed since 2006 when they introduced the version of their technology that is still the same today, after the U.S. Court of Appeals for the Federal Circuit directed the Federal District Court in Los Angeles to enjoin the Nellcor pulse oximeter that infringed Masimo's breakthrough measure-through motion patents. All studies we are aware of show that the current Nellcor technology does not match the performance of Masimo SET® technology during challenging motion conditions. We hope this $1,000,000 guarantee sends a clear message to clinicians, biomedical engineers, materials management, and hospital administrators to look to the objective evidence when they are interested in improving pulse oximetry performance."
Dr. Michael O'Reilly, Chief Medical Officer for Masimo, stated: "The ability of Masimo SET® to detect oxygen saturation changes (sensitivity) and avoid false alarms (specificity) is proven by more than 100 independent and objective studies, internal studies, and thousands of clinical evaluations. Most importantly, only Masimo SET® pulse oximetry has been shown to improve patient outcomes by helping clinicians reduce retinopathy of prematurity,4 screen newborns for critical congenital heart disease (CCHD),5,6 reduce ventilator weaning time,7 and save lives while reducing rapid response activations and intensive care unit transfers on the general floor."8
Dr. O'Reilly continued: "Since we introduced Masimo SET®, we have converted more than 2,600 hospitals worldwide. Yet, in the same period, fewer than one in 100 hospitals have switched from Masimo, and to the best of our knowledge, none did so due to performance. We understand that hospital conversions to a new technology require time and effort. We are so confident in the performance of Masimo technology and the difference it will make in the care of patients that we are willing to assure care providers that their time and efforts will not be wasted with our $1,000,000 guarantee."
Dr. Peter Cox, Clinical Director, Critical Care Medicine & Associate Chief Physician, Department of Critical Care Medicine at the Hospital for Sick Children in Toronto, stated: "Recently published reports support the notion that Masimo SET® pulse oximetry outperforms Nellcor pulse oximetry during motion and low perfusion conditions. Measure-through motion and low perfusion performance can be validated by changing the true arterial oxygen saturation during motion and low perfusion conditions. In the recently published study of SpO2 accuracy in both machine- and volunteer-generated motion, Masimo SET® had 97% sensitivity and 95% specificity while the Nellcor N-600 had 57% sensitivity and 72% specificity."9
Dr. Anne Granelli—lead author on one of the largest published CCHD studies to date, liaison to the U.S. Department of Health and Human Services Secretary's Advisory Committee on Heritable Diseases in Newborns and Children (SACHDNC) workgroup, and author of an award-winning doctoral thesis in this field—stated: "While working at one of the two centers in Sweden that performed pediatric cardiac surgery, I found a significant difference, when compared simultaneously with blood gas, between Masimo SET® and all other pulse oximetry technologies we used on cyanotic children in the PICU and pediatric cardiac ward. We were not aware of the significant difference between oximeters before this clinical study. And, as a result, we upgraded all our pulse oximeters to Masimo SET®."
Dr. Kenneth Rothfield, Chairman of Anesthesiology at Saint Agnes Hospital in Baltimore, stated: "In my 20 years' experience, I have used other pulse oximeters that claimed to tolerate motion, but this is easier said than done. When a patient's life hangs in the balance, a pulse oximeter that cannot measure despite motion and low perfusion is useless—and that can have dangerous consequences. That's one of the many reasons my hospital switched from Nellcor to Masimo SET®. Masimo SET® doesn't just tolerate motion, it measures through it – accurately and consistently."
Background
On May 20, 2013, Covidien announced FDA 510(k) clearance with motion claims for three Nellcor™ pulse oximeters and stated they are the "first company to receive FDA 510(k) clearance for a motion-tolerant bedside pulse oximeter portfolio that is compliant with ISO 80601-2-61," a new pulse oximetry testing standard.1 On May 28, 2013, Covidien announced that "Nellcor Pulse Oximeters Receive FDA 510(k) Clearance with Labeling for Use in Newborn Screening for Critical Congenital Heart Disease."2
Covidien's announcements may cause confusion because:
- Covidien did not disclose the specific motion claims for Nellcor pulse oximeters, and Covidien has not changed the motion performance of Nellcor pulse oximeters
The terms "motion claims" and "motion-tolerant" were not defined by Covidien and their test methods and results were not disclosed. However, any changes by Covidien in motion claims do not mean there have been any changes in motion performance. The motion performance of Nellcor pulse oximeters has not changed since 2006, when Nellcor introduced the N-600 and 06 circuit board, and then the N-600x, after U.S. courts found that previous Nellcor pulse oximeters infringed on multiple Masimo patents. The two Nellcor pulse oximeters introduced in 2012, the Bedside Monitoring System and Bedside Respiratory Monitoring Systems, share pulse oximetry technology with the 06/N-600/N-600x. - Covidien is not the first company to receive FDA 510(k) clearance for a pulse oximeter with motion claims while complying with ISO standards
Masimo was the first company to receive FDA 510(k) clearance with measure-through motion claims in 1998 and measure-through motion and low perfusion claims in 1999, and has always been in compliance with ISO standards. While the ISO standard 9919 is still in effect, compliance with the new ISO 80601 standard will be required by the FDA for all new FDA 510(k) submissions for pulse oximeters after June 30, 2013. The new ISO standard will also apply to CE marked products in 2014. The new ISO standard does not change how a pulse oximeter is tested under motion and low perfusion. - Covidien did not disclose the specific labeling for screening newborns for CCHD, and only Masimo SET® pulse oximetry has been proven in large studies to have high enough sensitivity and specificity to allow the clinical community to recommend CCHD screening
Any decisions on which pulse oximeter to use for newborn screening for CCHD screening should be made with protection of the patient and family as the highest priority. To maximize the effectiveness of newborn screening that is often performed in moving babies, use of pulse oximetry that can actually measure through motion is important to minimize both false negatives (failing to indicate a baby should receive diagnostic testing) and false positives (indicating a baby should receive diagnostic testing when they do not). After a thorough review, the CCHD workgroup that published the 2011 screening recommendations relied upon two independent, published studies,5,6 which included nearly 60,000 newborn subjects and exclusively used Masimo SET® pulse oximeters. The CCHD workgroup stated, "On the basis of new data from the large population-based screening activities in Sweden (Granelli) and England (Ewer), the work group developed a recommendation for screening that was based on what was shown to be effective in those studies." In contrast to the evidence for Masimo SET®, Masimo is unaware of any large studies using Nellcor pulse oximetry to screen newborns for CCHD that demonstrate similar effectiveness as shown by Drs. Granelli and Ewer. In fact, Covidien cites three studies using Nellcor pulse oximeters for newborn screening for CCHD with questionable results and/or negative conclusions.10,11,12 - Masimo SET® pulse oximetry provides clinically superior performance compared to Nellcor pulse oximetry during motion and is the only pulse oximetry shown to help clinicians improve patient outcomes.
Masimo SET® pulse oximeter technology takes venous blood movement into the assumption of the measurement and utilizes patented and proprietary algorithms to identify the venous blood signal, isolate it, and using five parallel algorithms including adaptive filtering, extract the arterial signal during all conditions, including motion and low perfusion. A video example of Masimo SET® and Nellcor motion performance during desaturation and resaturation can be viewed http://www.masimo.com/videos/Desat_Resat-100108.wmv. A video of Masimo SET® and Nellcor motion performance during low perfusion can be viewed http://www.masimo.com/videos/LP-100108.wmv. Masimo representatives can easily show the motion performance of both Masimo SET® and Nellcor pulse oximeters in a side-by-side demonstration. In addition, Masimo strongly encourages scientific, side-by-side evaluations with automated data collection on patients.
# # #
1 http://investor.covidien.com/phoenix.zhtml?c=207592&p=irol-newsArticle&ID=1821990&highlight=
2 http://investor.covidien.com/phoenix.zhtml?c=207592&p=irol-newsArticle&ID=1824373&highlight=
3 http://www.masimo.com/1millionrules.htm
4 Castillo A, et al. Acta Paediatr. 2011 Feb.;100(2):188-92.
5 de-Wahl Granelli A., et al. BMJ. 2009 Jan 8;338.
6 Ewer A, et al. Health Technol Assess. 2012;16(2):1-184.
7 Durbin, et al. Critical Care Medicine. 2002 Aug.;30(8): 1735 to 1740.
8 Taenzer A, et al. Anesthesiology, 2010 Feb;112(2):282-7.
9 Shah N, et al. J Clinical Anaesthesia. 2012 Aug;24(5):385-91.
10 Sendelbach DM, et al. Pediatrics. 2008 Oct. 122(4): p. e815-20.
11 Walsh, W. J Perinatol, 2011 Feb. 31(2): p. 125-9.
12 Reich, JD, et al. J Pediatr, 2003 Mar. 142(3):p. 268-72.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of published studies, internal clinical studies and side-by-side clinical evaluations; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
—end—
Media Contacts:
Mike Drummond
Masimo Corporation
(949) 297-7434
mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SedLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium. All other trademarks, service marks, trade names, logos and icons, registered or not, are the property of third parties as named/indicated.


El Paso Children's Hospital Standardizes to Masimo SET® Pulse Oximetry
Leading-Edge Texas Hospital Deploys Breakthrough Measure-Through Motion and Low Perfusion Pulse Oximetry Technology
El Paso, Texas & Irvine, California – May 23, 2013 – Masimo (NASDAQ: MASI) today announced that El Paso Children's Hospital, the newest and most expansive dedicated children's hospital in west Texas, has upgraded to Masimo SET® pulse oximetry and sensor technologies, the standard-of-care at leading health organizations worldwide.
Masimo SET® pulse oximetry has been shown to virtually eliminate false alarms1 and increase a clinician's ability to detect life-threatening events2 – helping to substantially contribute to improved patient outcomes and patient safety. The clinical accuracy of Masimo SET® pulse oximetry has also been shown to help clinicians significantly reduce retinopathy of prematurity3-5, screen for critical congenital heart disease in newborns6-8, reduce oxygen overdose9, 10 and medical errors11, 12, and save lives in post-surgical floors, recovery2, labor and delivery rooms13, and ICUs14.
El Paso Children's Hospital's conversion to Masimo SET® pulse oximetry is in keeping with the hospital's dedication to excellence in care and delivering the best possible results for its patients. More than 100 independent clinical studies have confirmed that Masimo SET® technology allows clinicians to accurately monitor blood oxygen saturation in the most challenging conditions, including patient movement and low peripheral perfusion.15
According to Eddie Pacheco, manager of respiratory care and neuro-diagnostics at El Paso Children's Hospital, "At times it's nearly impossible to obtain SpO2 readings – let alone accurate readings – with other types of pulse oximeters. I've found that with Masimo SET®, you get a quick accurate result, instead of waiting five minutes or more for a blood gas analysis to obtain an SaO2. This in turn benefits the children so they don't have to be poked for blood."
El Paso Children's also has the option to upgrade at any time to Masimo rainbow® Pulse CO-Oximetry™, which allows clinicians to noninvasively monitor multiple blood constituents, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™).
"We value the commitment and dedication of El Paso Children's Hospital clinicians and staff, who work hard every day on the front lines of healthcare to help children heal and recover to wellness," said Masimo founder and CEO Joe Kiani. "The performance of Masimo SET® pulse oximetry was not only designed with this patient population—prone to high motion and low perfusion—in mind, but was also designed to help reduce the burdens of false alarms. We are honored to partner with the clinicians at El Paso Children's Hospital to improve clinical outcomes and meet patient-care needs."
1 Shah N, Ragaswamy H, Govindugari K, Estanol L. "Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers." Journal of Clinical Anesthesia. 2012 (10.1016/j.jclinane.2011.10.012) https://www.sciencedirect.com
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Impact-of-Pulse-Oximetry-Surveillance-on-Rescue.
3 Chow L.C., Wright K.W., Sola A.; CSMC Oxygen Administration Study Group. "Can Changes in Clinical Practice Decrease the Incidence of Severe Retinopathy of Prematurity in Very Low Birth Weight Infants?" Pediatrics. 2003 Feb;111(2):339-45.
4 Clinical Practice and SpO2 Technology in the Prevention of ROP in VLBW Infants. Castillo AR, Deulofeut R, Sola A. Presented at Pediatric Academic Societies Annual Meeting May 5-8, 2007.
5 Oxygen as a neonatal health hazard: call for détente in clinical practice. Sola A., Rogido, Marta, Deulofeut, Richard. Acta Paediatrica 2007; 96:801-812.
6 de Wahl Granelli A, Wennergren M, Sandberg K, et al. Impact of pulse oximetry screening on detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ 2009; 338: a3037.
7 Noninvasive Peripheral Perfusion Index as a Possible Tool for Screening for Critical Left Heart Obstruction. Granelli, AD, Ostman-Smith, I. Acta Paediatrica 2007; 96:1455-1459.
8 Screening for Duct-Dependent Congenital Heart Disease with Pulse Oximetry: A Critical Evaluation of Strategies to Maximize Sensitivity. Granelli AD, Mellander M, Sunnegardh J,Sandberg K, Ostman-Smith I. Acta Paediatr 2005; 94:1590-6.
9 Avoiding Hyperoxemia During Neonatal Resuscitation: Time to Response to Different SpO2 Monitors. Baquero H, Alviz R, Sola A. Presented at Pediatric Academic Societies Annual Meeting May 5-8, 2007. A
10 More Reliable Oximetry Reduces the Frequency of Arterial Blood Gas Analyses and Hastens Oxygen Weaning after Cardiac Surgery. Durbin CG, Rostow SK. Crit Care Med 2002; 30(8): 1735-1740
11 Advantages of New Technology Pulse Oximetry with Adults in Extremis. Durbin CG, Rostow SK. Anesth Analg 2002; 94: S81-S83
12 Pulse Oximetry Performance Can Affect Caregiver Time Utilization. Durbin CG, Rostow SK. Anesthesiology 2000;93(3A): A556.
13 Baquero H, Alviz R, Castillo A, Neira F, Sola A. "Avoiding Hyperoxemia During Neonatal Resuscitation: Time To Response Of Different SpO2 Monitors." Acta Paed April 2011 Vol. 100, Issue 4, pp 515-518. Published online https://onlinelibrary.wiley.com/doi/full/10.1111/j.1651-2227.2010.02097.x
14 Goldstein MR, Martin GI, Sindel BD, Furman GI, Ochikubo C, Yand L. SatSeconds Alarm Management Misses Short Desaturations Common to Periodic Breathing and Infantile Apnea. Pediatric Research 2001;49(4):400A/2296.
15 Full Citation List of All Available Clinical Studies Featuring Masimo: http://www.masimo.com/cpub/clinical-masimo-set.htm
About El Paso Children's Hospital
located near the U.S./Mexico border, opened its doors to the public on February 14, 2012. El Paso Children's Hospital is the first and only separately licensed, independent, 501(c) (3) not-for-profit children's hospital in the El Paso region and is the only dedicated Pediatric Hospital within a 250-mile radius of El Paso, Texas. El Paso Children's Hospital is accredited by the Joint Commission and the College of American Pathologists. It is one of eight children's hospitals in the State of Texas and is a member of the Children's Hospital Association of Texas. El Paso Children's Hospital is one of 221 members of the Children's Hospital Association, and is the designated hospital in El Paso County for Children's Miracle Network. Additional information can be found at https://www.elpasochildrens.org.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the hospital-wide conversion ensures that all El Paso Children's Hospital patients will be cared for using the most technologically and clinically-advanced noninvasive patient monitoring solutions available; risks related to our belief that Masimo SET provides real-time results for all patients to help clinicians to more rapidly assess, diagnose, and treat every patient; risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb), SpCO, SpMet, PVI, and RRa contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Georgina M. Panahi
El Paso Children's Hospital
Phone: (915) 242-8354
Email: GPanahi@elpasochildrens.org
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.

Study Shows Noninvasive SpCO® Can Help Rapidly Detect Carbon Monoxide Poisoning in the Emergency Department
Irvine, California – May 21, 2013 – Masimo (NASDAQ: MASI) announced today that a new clinical study posted online in Respiratory Care confirms the clinical utility of Masimo's noninvasive carboxyhemoglobin (SpCO®) from rainbow® Pulse CO-Oximetry™ as a first-line assessment tool in helping clinicians rapidly detect carbon monoxide (CO) poisoning.1
CO poisoning accounts for an estimated 50,000 emergency department (ED) visits in the U.S. annually and is the leading cause of accidental poisoning death.2 Symptoms of CO poisoning include headache, dizziness, nausea/vomiting, confusion, fatigue, chest pain, shortness of breath, and loss of consciousness, but are often attributed to other illnesses such as the flu. Failure to diagnose CO poisoning can have disastrous consequences for patients and potentially other family members of the affected household.3 Unfortunately, only about half (50%) of U.S. acute care hospitals have laboratory CO-oximetry capabilities enabling confirmation of CO poisoning in the blood,4 likely due to the expense of the instrumentation. Additional delays occur if a patient needs hyperbaric oxygen therapy, which often requires transfer to yet another medical center with hyperbaric capability.
Previous studies have shown that SpCO can help clinicians increase CO poisoning detection by as much as 39%5 and is associated with a shorter time to initiation of treatment (4.4 vs. 5.3 hours) with hyperbaric oxygen.6 These data have previously led to suggestions that SpCO could be used as a tool to help determine whether an invasive blood measurement of carboxyhemoglobin (COHb) should be performed to confirm CO intoxication.7
Researchers at Departement des urgencies, Centre Hospitalier Regional Universitaire Lapeyronie in Montpellier, France, studied 93 consecutive patients suspected of CO exposure presenting to an urban-based university hospital ED. Patients were tested noninvasively with a Masimo Rad-57™ Pulse CO-Oximeter®, while simultaneous blood samples were taken for laboratory COHb analysis and comparison.
Diagnosis of CO poisoning was determined for 26 (28%) patients. Compared to laboratory COHb values, the bias and standard deviation of SpCO over all patients was -0.2% + 3.3%. In six subjects with very high COHb values (greater than 15%), the bias and standard deviation of SpCO was 1.2% + 2.2%. The area under the curve for SpCO's ability to detect CO poisoning, which takes into account both sensitivity and specificity, was 83% for non-smokers and 98% for smokers, with an optimal SpCO threshold of 6% for non-smokers and 9% for smokers.
Researchers concluded "noninvasive Pulse CO-Oximetry could be useful as a first-line screening test, enabling rapid detection and management of CO-poisoned patients in the ED."
1 Sebbane M, Claret PG, Mercier G, Lefebvre S, Thery R, Dumont R, et al. "Emergency Department Management of Suspected Carbon Monoxide Poisoning: Role of Pulse Co-Oximetry." Respir Care. Published online ahead of print Mar 19, 2013.
2 Hampson N., Weaver L. "Carbon Monoxide Poisoning: A New Incidence for an Old Disease." Undersea Hyperb Med 2007;34:163-168.
3 Hampson N., Piantadosi C., Thom S., Weaver L. "Practice Recommendations in the Diagnosis, Management, and Prevention of Carbon Monoxide Poisoning," American Journal of Respiratory and Critical Care Medicine, Vol. 186, No. 11 (2012), pp. 1095-1101.
4 Hampson NB, Scott KL, Zmaeff JL. "Carboxyhemoglobin measurement by hospitals: Implications for the diagnosis of carbon monoxide poisoning." J Emerg Med 2006;31(1):13-6.
5 Suner S, Partridge R, Sucov A, et al. "Noninvasive Pulse CO-Oximetry Screening in the Emergency Department Identifies Occult Carbon Monoxide Toxicity." J. Emerg Med. 2008; 34(4): 441-450.
6 Hampson N. "Noninvasive pulse CO-oximetry expedites evaluation and management of patients with carbon monoxide poisoning." The American Journal of Emergency Medicine 2012 (10.1016/j.ajem.2012.03.026)
7 Zaouter C, Zavorsky G. "The measurement of carboxyhemoglobin and methemoglobin using a noninvasive pulse CO-oximeter." Respiratory Physiology & Neurobiology July 2012.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SpCO will provide an accurate and effective noninvasive method of screening for CO poisoning, risks related to our belief that SpCO offers an effective front-line tool for first responders and emergency departments, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.

Masimo CEO Joe Kiani Receives Argyros Medal for Visionary Leadership & Commitment to Patient Safety
Chapman University's George L. Argyros School of Business and Economics Bestows Award at Commencement Address to Class of 2013
Irvine, California – May 20, 2013 – Chapman University's George L. Argyros School of Business and Economics awarded Joe Kiani, founder, CEO and Chairman of the Board of Masimo (NASDAQ: MASI), the Argyros Medal for his entrepreneurship, outstanding business accomplishments, service on behalf of patient safety, and dedication and support of Chapman University.
James L. Doti, President of Chapman University, and Reginald H. Gilyard, Dean of the Argyros School of Business and Economics, presented Kiani with the Argyros Medal before Joe Kiani delivered the Commencement Address to the Class of 2013.
Chapman officials noted that while English is his second language, Kiani graduated high school at the age of 15, and earned his Bachelor of Science and Master of Science degrees in electrical engineering at the age of 22.
"Joe demonstrates the meaning of hard work and resilience," said Gilyard, and added that with the knowledge gained through education, he founded Masimo, which "has grown exponentially through Joe's visionary leadership and is now a trailblazer in the medical technology field."
Gilyard also cited Kiani's passion and commitment for patient safety through his founding of the Patient Safety, Science & Technology Movement , with a goal to reduce preventable hospital deaths to zero by 2020, and his dedication to the Clinton Global Initiative to help alleviate maternal mortality and anemia in Sub-Sarah Africa starting with villages in Liberia and Uganda.
"Joe considers it his company's responsibility to make patient safety a top priority," Gilyard said. "And Masimo is working tirelessly to get medical decision-makers on board with this charge."
Stated Kiani: "I am truly humbled and honored to receive the Argyros Medal from Chapman University's George L. Argyros School of Business and Economics. As I noted in my Commencement Address to the Class of 2013, success should be defined in terms of happiness. This award represents a happy occasion, as well as a solemn reminder that ethical business practices and financial profits are not mutually exclusive."
About the George L. Argyros Award
The George L. Argyros Award is given every year on behalf of the Honorable George L. Argyros '59. This award is given at the annual Argyros School of Business and Economics' Commencement ceremony. The award is given to a member of the community who exemplifies entrepreneurship and service. The Argyros Medal honors the outstanding accomplishments of the Honorable George L. Argyros as an entrepreneur and leader in the business community, as well as his dedication and support for Chapman University. Please visit https://www.chapman.edu/asbe/about-george-argyros.aspx
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOCTM), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic MonitoringTM, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: whether Mr. Kiani's receipt of the Argyros Medal will have any material effect on our business, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5, Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.

New Clinical Study Finds Masimo rainbow®Acoustic Monitoring™ Technology More Accurately, Precisely, and Reliably Detects Ventilatory Pauses in Post-Surgical Patients
Published in the June 2013 Issue of Anesthesia & Analgesia, Study Demonstrates that Acoustic Respiration Rate (RRa™) is More Sensitive to Ventilation Changes than Capnometry
Irvine, California – May 16, 2013 – Masimo (NASDAQ: MASI) announced today that a new study to be published in the June 2013 edition of the journal Anesthesia & Analgesia (available online today) concludes that Acoustic Respiration Rate (RRa™) shows "significantly greater accuracy and precision for respiratory rate as compared to capnometry."1
Abnormal ventilatory rate is a common clinical occurrence that can precede a major clinical event, such as cardiac arrest, onset of sepsis, and respiratory infection.2,3 Continuous monitoring of respiration rate—a critical ventilatory vital sign that provides early detection of respiratory compromise and patient distress—is especially important for post-surgical patients receiving sedatives, opioids, or patient-controlled analgesia (PCA) for pain management as the sedation can induce respiratory depression and place patients at considerable risk of serious injury or death.4-7
In the published study, 33 (adult) post-surgical patients were monitored in the post-anesthesia care unit using a Masimo Pulse CO-Oximeter with rainbow®Acoustic Monitoring™ technology (Rad-87, version 7804) connected to an adhesive acoustic respiration rate Sensor (RAS-125, rev C) applied to the neck and a nasal cannula connected to a bedside capnometer (Oridion Capnostream20, version 4.5). Both the acoustic monitor and capnometer were connected to a computer for continuous acoustic and expiratory carbon dioxide waveform recordings—enabling automatic calculation of a reference ventilatory rate for each device—while a trained technician simultaneously listened to the breathing sounds from the acoustic signal to determine inspiration and expiration reference markers within the ventilatory cycle. Results after 3,712 total monitoring minutes (average of 112 minutes per subject) showed rainbow®acoustic respiration rate monitoring had "significantly greater accuracy (P = 0.0056) and precision (P = 0.0024) for respiration rate as compared with capnometry" and "trended a higher sensitivity (P = 0.0461)" to pauses in ventilation (81% vs 62%) in 21 apneic events." While reliability of the two devices was high on average, study authors noted that study "results suggest that the capnometer is about 3% less precise ."1
 Masimo rainbow®Acoustic Monitoring™ technology features an adhesive acoustic respiration rate (RRa™) sensor applied to the patient's neck to continuously measure respiration rate. |
The study concluded that, relative to capnometry, acoustic respiration rate (RRa) "demonstrated greater accuracy, precision, and sensitivity to pauses in ventilation." According to the study's lead researcher and author, Michael Ramsay, M.D., Chief of the Department of Anesthesiology and Pain Management at Baylor University Medical Center in Dallas, "The results of our study show that the addition of RRa for continuous ventilatory rate monitoring provides a safety net for post-surgical patients receiving opioids with the potential to greatly improve the timeliness of response to an adverse respiratory event. Both of which help to improve patient safety and outcomes for post-surgical patients in recovery areas and on general care floors."
Masimo rainbow®Acoustic Monitoring™ technology noninvasively and continuously measures respiration rate using an innovative adhesive sensor with an integrated acoustic transducer that is easily and comfortably applied to the patient's neck. Using acoustic signal processing that leverages Masimo's patented revolutionary Signal Extraction Technology (SET®), the respiratory signal is separated and processed to display continuous acoustic respiration rate (RRa)—enabling earlier detection of respiratory compromise and patient distress.
As a result of mounting evidence of the increased risk to patients, many nationally recognized health care organizations have launched major initiatives recommending continuous monitoring of oxygenation and ventilation of post-surgical patients to detect and respond appropriately to the early indicators of physiological deterioration, including: the Institute for Healthcare Improvement,8 the Joint Commission,9,10 the Anesthesia Patient Safety Foundation,11 and the American Society of Anesthesiologists.12 Traditional methods for monitoring a patient's respiration rate have demonstrated limitations that can affect accuracy and patient tolerance.13-15
"This study provides a side-by-side analysis of RRa and capnography measurements that compares more than 3,700 monitored minutes of continuous respiration data," said Michael O'Reilly, M.D., Chief Medical Officer of Masimo. "With such detailed data points and critical analysis, the results are undeniable—Masimo rainbow®Acoustic Monitoring™ technology offers improved sensitivity, accuracy, precision and reliability over capnometry for continuous monitoring of ventilation rate in post-surgical patients."
1 Ramsay M, Usman M, Lagow E, Mendoza M, Untalan E, De Vol E. "The Accuracy, Precision and Reliability of Measuring Ventilatory Rate and Detecting Ventilatory Pause by rainbow Acoustic Monitoring and Capnometry." Anesth Analg; April 30, 2013 ANE.0b013e318290c798.
2 Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. "Clinical antecedents to in-hospital cardiopulmonary arrest." Chest 1990;98:1388–92.
3 Mailey J, Digiovine B, Baillod D, Gnam G, Jordan J, Rubinfeld I. "Reducing hospital standardized mortality rate with early interventions." J Trauma Nurs 2006;13:178–82.
4 Joint Commission on Accreditation of Healthcare Organizations. Sentinel event alert: patient controlled analgesia by proxy; JCAHO. 2004.
5 Institute for Safe Medication Practices. Safety issues with patient-controlled analgesia: Part I – How errors occur; ISMP. 2003.
6 Institute for Safe Medication Practices. Safety issues with patient-controlled analgesia: Part II – How to prevent errors; ISMP. 2003.
7 Bird M. "Acute pain management: a new area of liability for anesthesiologists"; ASA Newsletter. 2007; 71:8.
8 Institute for Healthcare Improvement. Rapid Response Teams.
9 The Joint Commission. 2009 National Patient Safety Goals.
10 The Joint Commission. Sentinel event alert: Safe use of opioids in hospitals; 2012.
11 Anesthesia Patient Safety Foundation. APSF Newsletter. "No Patient Shall be Harmed by Opioid-Induced Respiratory Depression." 2011; 26(2):21-40.
12 American Society of Anesthesiologists. Practice Guidelines for the Prevention, Detection and Management of Respiratory Depression Associated with Neuraxial Opioid Administration
13 Maddox RR, Williams CK, Oglesby H, Butler B, Colclasure B. "Clinical experience with patient-controlled analgesia using continuous respiratory monitoring and a smart infusion system." Am J Health Syst Pharm 2006;63:157–64.
14 Friesen RH, Alswang M. "End-tidal PCO2 monitoring via nasal cannulae in pediatric patients: accuracy and sources of error." J Clin Monit 1996;12:155–9.
15 Gaucher A, Frasca D, Mimoz O, Deboene B. "Accuracy of respiratory rate monitoring by capnometry using the Capnomask in intubated patients receiving supplemental oxygen after surgery." Br. J. Anaesth 2012;108:316–20.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results and performance of Masimo rainbow Acoustic Monitoring; our belief that the breakthrough acoustic respiration rate (RRa) capabilities of Masimo's proprietary rainbow acoustic monitoring technology will provide more accurate, precise, and reliable results over capnography in all patients and monitoring conditions – enabling clinicians to detect and treat respiratory compromise and patient distress earlier; and other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


New Clinical Studies Presented at the International Anesthesia Research Society Annual Meeting Show Benefits of Masimo Noninvasive Patient Monitoring Technologies: SpHb®, RRa™, and SedLine®
Irvine, California – May 13, 2013 – Masimo (NASDAQ: MASI) announced today that three new clinical studies evaluating Masimo noninvasive patient monitoring technologies were presented before the world's leading anesthesia educators and investigators at the International Anesthesia Research Society (IARS) Annual Meeting in San Diego. The following studies highlight the positive clinical outcomes and patient safety impact of Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®), acoustic respiration rate (RRa™), and SedLine®brain function monitoring.
Total Hemoglobin (SpHb)
Researchers at the Resuscitation Research Laboratory at the University of Texas Medical Branch measured SpHb continuously with a Masimo Radical-7 Pulse CO-Oximeter®, and compared measurements with total hemoglobin (tHb) by arterial blood sampling in volunteer patients under general anesthesia. Researchers said, "SpHb lends itself to effective trend analysis and accurate assessment of changes in tHb during periods of significant hemodilution. Trending was highly reliable in terms of concordance and trending ability."1
rainbow®Acoustic Monitoring for RRa and SedLine
Researchers at Loma Linda University School of Medicine used standard monitoring of patients during procedural sedation, which included pulse oximetry (SpO2) and noninvasive blood pressure, in addition to rainbow®acoustic monitoring (RRa, Rad-87 Pulse CO-Oximeter®), and depth of sedation monitoring using Patient State Index (PSI™) and SedLine. They found, "Respiratory depression, apnea and deep sedation were common in our cohort of patients receiving procedural sedation. These events may not be detected by standard care monitoring of SpO2 and blood pressure. We found concurrent excess sedation or respiratory depression with SpO2 =92% in some patients. Our results indicate that advanced respiration rate and brain function monitoring should be considered for patients undergoing procedural sedation."2
In another study, researchers David R. Drover, M.D., and Pedro Tanaka, M.D., at Stanford University School of Medicine, evaluated results of patients who underwent total knee arthoplasty and were provided monitored anesthesia care (MAC) – a combination of sedation and/or analgesic drugs common for less invasive procedures, with the benefit of fewer side effects such as respiratory depression and apnea. Patients received routine ASA monitoring as well as measurement of respiration rate by rainbow®acoustic monitoring (RRa, Rad-87) and depth of sedation measured by PSI and SedLine. Researchers concluded: "Both apnea and excessive sedation occur in patients undergoing MAC but do not occur at the same time. This is consistent with our knowledge of the anatomy of the upper airway and risks of airway obstruction. This suggests that both respiration rate monitoring for the detection of hypoventilation and brain function monitoring for depth of sedation are indicated for patients being provided sedation."3
1 Marques N, Kramer G, Salter M, Voigt R, Kinsky M. "Trending, Accuracy and Precision of Noninvasive Hemoglobin Monitoring During Hemodilution." Proceedings of the International Anesthesia Research Society, May 7, 2013. San Diego. S-386
2 Applegate R, Macknet M, Qoshlli S, Mehdizadeh A, Jacobson P, Neumann M, Allard M. "Incidence of Deep Sedation and Respiratory Compromise During Procedural Sedation" Proceedings of the International Anesthesia Research Society, May 7, 2013. San Diego. S-298
3 Drover D, Tanaka P. "Depth of Sedation Does Not Predict Episodes of Apnea." Proceedings of the International Anesthesia Research Society, May 7, 2013. San Diego. S-302
*To see a summary of all known clinical studies and abstracts on Masimo technologies and noninvasive measurements, please visit: http://www.masimo.com/cpub/clinicals.htm.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET®outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow®Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET®and Masimo rainbow®technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®), acoustic respiration rate (RRa™), and SedLine®contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.



Uludag University Hospital Installs Masimo Noninvasive rainbow®Technology to Improve Patient Assessments and Speed Clinical Decisions
Use of SpHb®Noninvasive Hemoglobin Is Part of Leading Medical Teaching Facility's Standardization to Masimo SET®Pulse Oximetry
Bursa, Turkey & Irvine, California – May 8, 2013 – Masimo (NASDAQ: MASI) and Uludag University Hospital, one of the top educational healthcare facilities in all of Turkey, announce that Uludag has added Masimo SpHb®– a breakthrough technology that allows clinicians to noninvasively and continuously monitor hemoglobin blood levels.
The use of SpHb is part of Uludag's standardization to Masimo SET®Measure-Through Motion and Low Perfusion™ pulse oximetry, clinically shown to virtually eliminate false alarms1 and help clinicians better detect life-threatening events.2 This conversion represents Masimo's largest whole-house technology installation in Turkey to date.
Uludag University Hospital, with 880 beds and only one of two hospitals in the country to have Joint Commission International accreditation, is now leveraging Masimo SpHb in its operating rooms. SpHb has been clinically shown to reduce risky and unnecessary blood transfusions and speeds time to transfuse when indicated, while dramatically reducing costs.3
"We use the Masimo Radical-7 Pulse CO-Oximeter with SpHb primarily for complex neurosurgeries of pediatric patients such as brain tumors and craniosynostosis," said Dr. Gülsen Korfali, a professor and Chairman of Anesthesiology and Reanimation at Uludag University. "We have a particular need to track hemoglobin levels on a continuous basis and stay noninvasive on the most vulnerable patient population – children."
"Masimo SET also has tremendously reduced our sensor consumption because they last longer," Dr. Korfali added. "We used to discard sensors every day. Now we can use a sensor for up to five days. We also have almost no false alarms and a higher ability to detect desaturations with Masimo SET in our critical care areas."
Hemoglobin levels are used as a primary indicator for red blood cell (RBC) transfusion, but conventional laboratory measurements are only available intermittently and results can be delayed in the period between blood draw and laboratory analysis. This time gap of information can lead to sub-optimal transfusion decisions.4 RBC transfusion overuse as well as delayed RBC transfusion can increase patient risk and cost of care. Multiple observational studies have shown that patients receiving RBC transfusions have an 88% higher mortality, 69% higher infection rate, and 250% higher rate of acute respiratory distress syndrome.5
SpHb monitoring provides real-time directional trends in hemoglobin, such as indicating stable hemoglobin when it may be perceived to be dropping, and rising hemoglobin when it may be perceived to not be rising fast enough. A recent study from Cairo University in Egypt also showed that once clinicians determined a transfusion was needed, they were able to initiate transfusions 82% faster – in about 9 minutes, compared to about 50 minutes for patients not being monitored by SpHb.3 That same study also showed SpHb can help clinicians reduce unnecessary and risky RBC transfusions, which can improve patient outcomes while dramatically lowering the cost of care.
Joe Kiani, founder and CEO of Masimo, stated: "We are honored that Uludag University Hospital, one of the top-performing hospitals in Turkey with an inspiring reputation for excellence, has selected Masimo for its patient monitoring needs. As a forward-thinking healthcare organization that embraces innovative medical technologies, we are eager to help Uludag improve its already superb patient safety and outcomes."
1 Shah N, Ragaswamy H, Govindugari K, Estanol L. "Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers." Journal of Clinical Anesthesia. 2012 (10.1016/j.jclinane.2011.10.012) https://www.sciencedirect.com
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Impact-of-Pulse-Oximetry-Surveillance-on-Rescue.
3 Awada W.F.N., Maher F. Reduction in Red Blood Cell Transfusions during Neurosurgery with Noninvasive and Continuous Hemoglobin Monitoring. Proceedings of the Society for Technology in Anesthesia Annual Meeting ; 2013 Jan 9-12; Phoenix AZ.
4 Friedman MT et al. Arch Pathol Lab Med. 2006 Apr;130(4):474-9.
5 Marik PE.et.al. Crit Care Med. 2008;36(9):2667-74
About Uludag University Hospital
Uludag University Hospital, with 880 beds and only one of two hospitals in Turkey to have Joint Commission International accreditation, is now leveraging Masimo SpHb in its operating rooms and Intensive Care Units. Please visit https://uludag.edu.tr/english/
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo SET has been shown to virtually eliminate false alarms and help clinicians better detect life-threatening events; risks related to our belief that Masimo's unique noninvasive SpHb measurement allows clinicians to noninvasively and continuously monitor hemoglobin blood levels, reduces risky and unnecessary blood transfusions, speeds necessary blood transfusions, dramatically reduces costs, and contributes to positive clinical outcomes and patient safety in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Prof. Dr. Gülsen Korfali
Uludag University Hospital
Phone: +90 5337460092
Email: sen@Uludag .edu.tr
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


FDA Clears Masimo rainbow® Acoustic Monitoring™ Sensor for Use on Pediatric Patients
Acoustic Respiration Rate (RRa™) Now Offers Accuracy,
Ease of Use and Enhanced Tolerance for Pediatric Patients
Irvine, California – May 6, 2013 – Masimo (NASDAQ: MASI) announced today that its rainbow® Acoustic Monitoring™ sensor, RAS-125c Acoustic Respiration Cloth Sensor has received U.S. FDA 510(k) clearance for continuous, noninvasive monitoring of acoustic respiration rate (RRa™) in pediatric patients. Previously FDA-cleared for adult use, clinicians can now use the RAS-125c Acoustic Respiration Cloth Sensor to noninvasively and continuously assess breathing acoustically in both adult and pediatric patients.
 Masimo RAS-125c acoustic respiration rate (RRa) sensor is for pediatric patients. |
Respiration rate is a critical vital sign that provides early detection of respiratory compromise and patient distress. Continuous monitoring of respiration rate is especially important for post-surgical patients receiving patient-controlled analgesia (PCA) for pain management as the sedation can induce respiratory depression and place patients at considerable risk of serious injury or death.1-4 Last year The Joint Commission issued a Sentinel Event Alert on opioid-induced respiratory depression and recommended continuous monitoring of oxygenation and ventilation of post-surgical patients.5 Similarly, Anaesthesia Patient Safety Foundation (APSF) guidelines include oxygenation and ventilation monitoring in all patients receiving opioids.6 Historically, traditional methods for respiration rate monitoring—nasal cannula capnography—can be limited by reliability and patient tolerance.7
Masimo rainbow® Acoustic Monitoring noninvasively and continuously measures respiration rate using an innovative adhesive sensor with an integrated acoustic transducer that is easily and comfortably applied to the patient's neck. Now indicated for use in pediatric patients, the accuracy of the RAS-125c Acoustic Respiration Rate Sensor remains the same (as the adult version) at ±1 over the range of 4 to 70 breaths per minute.
"It's going to be a home run," Michael Ramsay, M.D., Chief of the Department of Anesthesiology and Pain Management at Baylor University Medical Center in Dallas, said of RRa. "Kids don't tolerate capnography," which entails a nasal cannula for monitoring respiratory rate and end-tidal CO2. Ramsay recently finished a study on the efficacy of RRa that will soon be published in Anesthesia & Analgesia.8
"We have been eager to offer this patient-friendly respiration rate monitoring solution to pediatric patients in the United States," said Masimo Founder and CEO Joe Kiani. "We believe that noninvasive, continuous monitoring of acoustic respiration rate is better suited for younger patients than traditional capnography technologies that we also offer. And because the RAS-125c Acoustic Respiration Rate Sensor is more comfortable and tolerable for patients, it will help clinicians improve patient outcomes."
1 Joint Commission on Accreditation of Healthcare Organizations. Sentinel event alert: patient controlled analgesia by proxy; JCAHO. 2004.
2 Institute for Safe Medication Practices. Safety issues with patient-controlled analgesia: Part I – How errors occur; ISMP. 2003.
3 Institute for Safe Medication Practices. Safety issues with patient-controlled analgesia: Part II – How to prevent errors; ISMP. 2003.
4 Bird M. Acute pain management: a new area of liability for anesthesiologists; ASA Newsletter. 2007; 71:8.
5 The Joint Commission. Sentinel event alert: Safe use of opioids in hospitals; 2012
6 Weinger MB et al. APSF Newsletter. 2011; 26(2):21-40.
7 Macknet MR, et al. Accuracy and tolerance of a novel bioacoustic respiratory sensor in pediatric patients; Anesthesiology. 2007; A84.
8 Ramsay M, Usman M, Lagow E, Mendoza M, Untalan E, De Vol E. "The Accuracy, Precision and Reliability of Measuring Ventilatory Rate and Detecting Ventilatory Pause by rainbow Acoustic Monitoring and Capnometry." Anesth Analg; April 30, 2013 ANE.0b013e318290c798; available ahead of print online http://www.anesthesia-analgesia.org/content/early/2013/04/30/ANE.0b013e318290c798.full.pdf+html.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET®outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results and performance of Masimo rainbow Acoustic Monitoring; our belief that the breakthrough acoustic respiration rate monitoring capabilities of Masimo's proprietary RRa technology will provide sufficient sensitivity and specificity to measure respiratory rate in a variety of patients and monitoring conditions--enabling clinicians to detect and treat respiratory compromise and patient distress earlier; as well as assumptions regarding full commercialization timing and worldwide market availability of the technology; and other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo Reports First Quarter 2013 Financial Results
Q1 2013 Highlights (compared to Q1 2012):
- Total revenue, including royalties, rose 14% to $135.9 million
- Product revenue rose 15% to $128.6 million
- Masimo rainbow revenue rose 24% to $10.5 million
- Masimo SET®and Masimo rainbow®SET unit shipments rose 19% to 39,500
- Net income rose to $16.4 million, with EPS of $0.28 versus $0.27 in the year-ago period
Irvine, California, May 2, 2013 – Masimo (NASDAQ: MASI) today announced its financial results for the first quarter ended March 30, 2013.
Masimo's total first quarter revenue, including royalties, rose 14% to $135.9 million, compared to $119.2 million for the first quarter of 2012. First quarter 2013 product revenue rose 15% to $128.6 million, compared to $112.2 million for the first quarter of 2012. The company's worldwide direct product revenue grew 13% in the first quarter of 2013 and represented 84% of product revenue. OEM sales, which accounted for 16% of product revenue, rose 26% compared to the same period in 2012. Revenue from sales of Masimo rainbow products rose 24% to $10.5 million in the first quarter, compared to $8.5 million for the first quarter of 2012.
Net income for the first quarter of 2013 was $16.4 million, or $0.28 per diluted share, compared to net income of $15.8 million, or $0.27 per diluted share, in the first quarter of 2012. First quarter 2013 earnings per share were reduced by approximately $0.03 due to non-operating expenses primarily related to realized and unrealized net losses on foreign currency denominated transactions due to the strengthening of the U.S. dollar against the Japanese yen.
During the first quarter, the company shipped approximately 39,500 Masimo SET pulse oximetry and Masimo rainbow SET Pulse CO-Oximetry units, excluding handheld units, up 19% compared to approximately 33,300 in the same prior-year period. Masimo estimates its worldwide installed base as of March 30, 2013 to be 1,117,000 units, up 11% from 1,005,000 units as of March 31, 2012.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "Masimo is off to a solid start in 2013, with product revenue up 15%, fueled by a 14% rise in our core SET Pulse Oximetry business and a 24% rise in sales of rainbow SET Pulse CO-Oximetry and other rainbow related products. The 19% increase in driver shipments in the quarter further underscores the growing demand for our superior technology in hospitals and alternate care settings worldwide. In addition, we took advantage of strong cash flows in the quarter to repurchase 778,000 shares of Masimo common stock, demonstrating our confidence in the company's strategy, business model and future growth prospects."
As of March 30, 2013, Masimo's cash and cash equivalents were $81.6 million, compared to $71.6 million as of December 29, 2012. The change reflects primarily net cash generated from operations, offset by $12.4 million in cash used to repurchase approximately 778,000 shares of Masimo common stock.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 32475273. After the live webcast, the call will be available on Masimo's website through June 2, 2013. In addition, a telephonic replay of the call will be available through May 16, 2013. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 32475273.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET®outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. And in 2012, Masimo acquired the assets of Spire Semiconductor, LLC, a maker of advanced light emitting diode (LED) and other advanced component-level technologies; and PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies; and demand for our technologies. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the integration of acquisitions; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
# # #
Investor Contact:
Sheree Aronson
(949) 297-7434
saronson@masimo.com
Media Contact:
Mike Drummond
(949) 297-7043
mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.
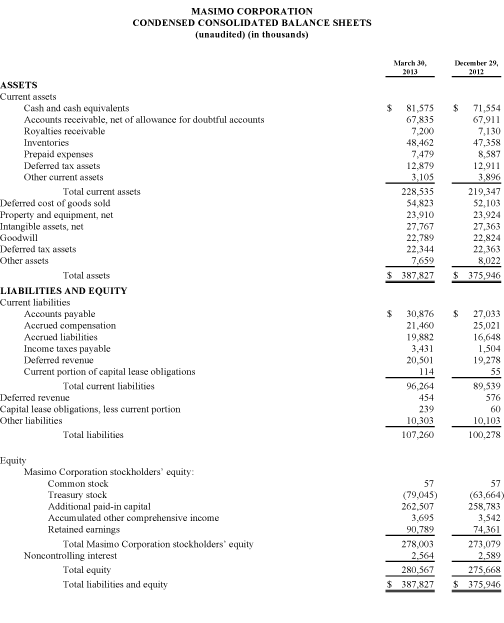

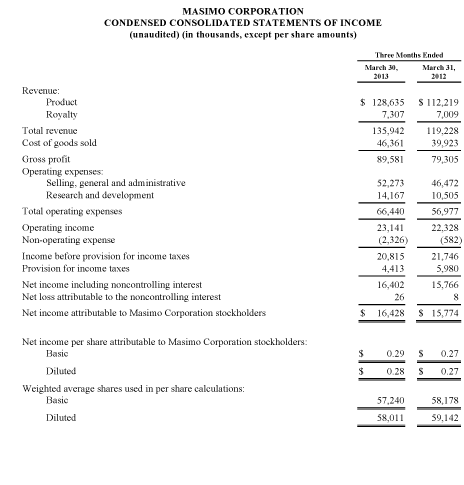



Masimo iSpO2 Pulse Oximeter & EMMA Emergency Capnometer Earn JEMS Hot Products Awards
at EMS Today 2013 Conference & Exposition
Masimo One of Two Companies (out of 300) to Win Two Awards at 31st Annual Industry-Leading Emergency Medical Services Event
 EMMA Mainstream Capnometer™ continuously measures, displays, and monitors respiratory rate and end-tidal carbon dioxide (EtCO2).
|

Masimo iSpO2™ pulse oximetry cable and sensor (available at iSpO2.com ) is compatible with the iPhone, iPad, or iPod touch. |
Irvine, California – April 29, 2013 – Masimo (NASDAQ: MASI) announced today that its iSpO2™ pulse oximeter for iPad, iPhone, and iPod touch and EMMA™ Emergency Capnometer earned JEMS Hot Product awards. Only two companies received dual Hot Product award honors at the EMS Today 2013 Conference & Exposition, the industry's leading emergency medical services (EMS) event held recently in Washington, D.C.
A dozen judges reviewed and evaluated products that had recently been introduced into the emergency service industry by the more than 300 exhibitors at the EMS Today Conference & Exhibition and rated each of them on originality, functionality, ease of use, and need in the EMS setting. The top 25 products were then selected as the hottest new products shown at EMS Today 2013.
"This year just two companies got two products selected in the top 25 – quite an achievement," said AJ Heightman, editor-in-chief of JEMS. "We have had very few double winners in the past."
The EMMA Mainstream Capnometer measures, displays, and monitors respiratory rate and end-tidal carbon dioxide (EtCO2) continuously when connected to a patient's breathing circuit. EMMA's portability allows for use during cardiopulmonary resuscitation (CPR) and intubation in multiple points of care including pre-hospital, rapid response, emergency medicine, operating room, intensive care unit, and long-term acute care. Because EMMA is integrated into the breathing circuit for easy viewing during CPR and endotracheal tube placement, it is highly accessible during transport and/or emergency ventilation scenarios—allowing quick assessment in just a few seconds.
The Masimo iSpO2™ is a consumer pulse oximeter utilizing Masimo SET®technology – the same technology used in leading hospitals worldwide, providing accurate measurements even during the challenging conditions of motion and low perfusion – for use with iPhone, iPad or iPod touch with 30-pin connector to noninvasively measure blood oxygenation, pulse rate and perfusion index. iSpO2 is for short-term sports and aviation use and is not intended for medical use.
"We are honored that our iSpO2 pulse oximeter and EMMA capnometer received two prestigious JEMS Hot Products Awards at EMS Today, the preeminent conference and exhibition for the emergency services industry," said Jon Coleman, Masimo President of Worldwide Sales and Marketing and Clinical Research. "Masimo has always been committed to taking monitoring technologies to new sites and applications, and we're equally committed to launching more award-winning products."
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET®and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to the performance of iSpO2 with Masimo SET technology and its ability to provide accurate measurements even during the challenging conditions of motion and low perfusion; risks related to the performance of EMMA and its ability to provide quick assessment in just a few seconds in all cases, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


Masimo Steps Up with March for Babies™ to Fight for Healthier Newborns
March of Dimes Calls on Global Community to Walk, Donate
Irvine, California – April 26, 2013 – Masimo (NASDAQ: MASI) will lead the annual March of Dimes March for Babies Walk beginning 7 a.m., Sunday, April 28, at Fashion Island, Newport Beach, when thousands of volunteers and donors will join the fight against birth defects, premature birth, and infant mortality.
"Newborns are among the most vulnerable in any society," said Joe Kiani, CEO of Masimo and Chair of the Orange County March of Dimes 2013 March for Babies. "We owe it to them to create a safe, nurturing and healthy entrance to the world. Together we can help eliminate preventable birth defects and infant deaths."
It's not too late to register to walk, and those unable to attend can donate from anywhere in the world. Please visit https://www.marchforbabies.org/team/masimo .
The March of Dimes' mission to improve the health of babies aligns with Masimo's long-standing goal to help clinicians screen for critical congenital heart disease (CCHD) and reduce retinopathy of prematurity (ROP), a devastating eye disorder resulting in partial or complete blindness in premature newborns.
The March of Dimes was instrumental in passage last year of a California law requiring general acute care hospitals with licensed perinatal services to offer pulse oximetry screening for CCHD, a group of life-threatening heart defects that require intervention within the first days or year of life. CCHD causes up to 3% of all infant deaths in the first year of life.1 According to the U.S. Department of Health and Human Services (HHS), these types of heart defects affect about 7 to 9 of every 1,000 live births, one quarter of which could be detected and potentially treated by measuring blood oxygen saturation.
Masimo's pulse oximetry technology – the first to achieve accurate monitoring results with squirming babies and those with low perfusion (blood-flow) to their extremities – helps clinicians screen for potential CCHD and the need for additional testing before a baby leaves the hospital. Masimo's technology played a key role informing HHS's decision in 2011 to add CCHD screening to the national uniform screening panel, spurring a groundswell of recently introduced and enacted state CCHD screening laws across the country.
"We're thrilled to have Joe Kiani and Masimo on board to support us at a local level," said Celia Wheeler, Executive Director of the Orange County Division of March of Dimes. "His passion for the cause and commitment to helping our babies will be a huge driving factor in the success of our event this year. We are grateful for the time and support Mr. Kiani has been able to lend us."
1. Secretary of Health & Human Services letter to the Secretary's Advisory Committee on Heritable Disorders in Newborns and Children (SACHDNC); dated September 21, 2011.
About the March of Dimes
The March of Dimes is the leading nonprofit organization for pregnancy and baby health. With chapters nationwide and its premier event, March for Babies®the March of Dimes works to improve the health of babies by preventing birth defects, premature birth and infant mortality. For the latest resources and information, visit marchofdimes.com, nacersano.org, facebook.com/marchofdimesca, and twitter.com/modcal.
About the March for Babies
March for Babies is the March of Dimes annual fundraising event to help every baby have a healthy start. March for Babies is the March of Dimes largest fundraiser. It's a family-friendly event where the community comes together to walk for healthy babies. Money raised through March for Babies supports lifesaving programs of research, community outreach, education and advocacy. One day, all babies will be born healthy – but we need to walk to get there. Information about March for Babies can be found at marchforbabies.org.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET®outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET®and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo SET provides real-time results for all patients to help clinicians to more rapidly assess, diagnose, and treat every patient with CCHD; helps reduce ROP; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Sheri Lunn
March of Dimes
Phone: (818) 539-2191
Email: slunn@marchofdimes.com
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo to Report First Quarter 2013 Financial Results after Market Close on Thursday, May 2
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., April 18, 2013 -- Masimo (NASDAQ: MASI) announced today that it will release first quarter 2013 financial results for the period ended March 30, 2013, after the market closes on Thursday, May 2, 2013. The conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer. A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 32475273. After the live webcast, the call will be available on Masimo's website through June 2, 2013. In addition, a telephonic replay of the call will be available through May 16, 2013. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 32475273.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET®outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. And in 2012, Masimo acquired the assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Contact:
Sheree Aronson
(949) 297-7043
saronson@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.



East Tennessee Children's Hospital Installs Masimo Patient SafetyNet™ System for Improved Oversight of Postoperative Patients
Installation Is Part of the Organization-Wide Conversion to Masimo SET® Pulse Oximetry and Use of rainbow® Pulse CO-Oximetry™ for Advanced Patient Monitoring
Knoxville, Tennessee & Irvine, California – April 5, 2013 – East Tennessee Children's Hospital, one of four hospitals in Tennessee to be certified as a Comprehensive Regional Pediatric Center, and Masimo(NASDAQ: MASI) today announced that the hospital has deployed Masimo Patient SafetyNet™, clinically shown to help improve patient outcomes and save money.1
The installation at East Tennessee Children's is part of the leading pediatric healthcare organization's standardization to Masimo SET® Measure-Through Motion and Low Perfusion pulse oximetry and rainbow®Pulse CO-Oximetry
"We are excited about the extra layer of safety for our postoperative patients for inpatient surgery," said Debi Dobbs, Nurse Manager at East Tennessee Children's Hospital. "It's especially beneficial for those patients who suffer from respiratory depression, and we are exploring placing the Patient SafetyNet system in additional units."
East Tennessee Children's joins a growing list of prominent health systems using Patient SafetyNet, which can help ensure patients' safety by noninvasively and continuously measuring and tracking their underlying physiological conditions and detect changes or abnormalities that signal declining health status in real-time. When changes occur in the measured values, which may indicate deterioration in the patient's condition, the system automatically sends wireless alerts directly to clinicians – prompting a potentially lifesaving response to the patient's bedside. Patient SafetyNet also has been clinically shown to reduce preventable and costly rescue events and transfers to intensive care units.1
In addition, East Tennessee Children's Hospital patients will benefit from breakthrough rainbow® technology that allows clinicians to noninvasively measure multiple blood constituents, respiration rate, and other physiological parameters. Parameters in use at East Tennessee Children's include total hemoglobin (SpHb®)in operating rooms, in addition to the Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR). The pulse oximetry standard-of-care at leading hospitals worldwide, Masimo SET® virtually eliminates false alarms2 and increases a clinician's ability to detect life-threatening events.3
Masimo's SpHb enables clinicians to monitor hemoglobin and trending noninvasively and continuously,4 and has been clinically shown to help anesthesiologists reduce the frequency of unnecessary blood transfusions,5 which carry risks that include a significant link to mortality and infection.6
"There is no higher calling in medicine than to offer the best care possible for children, and East Tennessee Children's Hospital has shown that caring for children is its top concern," said Joe Kiani, founder and CEO of Masimo. "We are delighted to be partnering with East Tennessee Children's Hospital to help protect young patients, improve outcomes, and reduce costs."
- Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012.
- Shah N, Ragaswamy H, Govindugari K, Estanol L. "Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers." Journal of Clinical Anesthesia. 2012 (10.1016/j.jclinane.2011.10.012) Available online https://www.sciencedirect.com
- Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study. Anesthesiology, February 2010, Vol. 112, Issue 2. Available online Impact-of-Pulse-Oximetry-Surveillance-on-Rescue.
- Frasca D., Dahyot-Fizelier C., Catherine K., Levrat Q., Debaene B., Mimoz O. "Accuracy of a Continuous Noninvasive Hemoglobin Monitor in Intensive Care Unit Patients." Crit Care Med. 2011 Oct;39(10):2277-82.
- Ehrenfeld JM, Henneman JP, Sandberg WS. "Impact of Continuous and Noninvasive Hemoglobin Monitoring on Intraoperative Blood Transfusions." American Society Anesthesiologists. 2010;LB05
- Marik, P. E. and H. L. Corwin (2008). "Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature."Crit Care Med 36(9): 2667-74.
About East Tennessee Children's Hospital
As a Comprehensive Regional Pediatric Center, East Tennessee Children's Hospital offers full capabilities to care for seriously ill children in a unique pediatric environment, as well as offering the services of many different pediatric subspecialties. East Tennessee Children's Hospital works jointly with the University of Tennessee Medical Center to ensure that injured children in this region receive trauma care at the institution most appropriate for the child's needs. Comprehensive centers also are responsible for assisting smaller hospitals to meet the requirements of their selected designations, primarily by providing pediatric health care training opportunities to these hospitals. East Tennessee Children's Hospital was the first hospital in East Tennessee to be certified by the state as a Comprehensive Regional Pediatric Center. For more information, please visit https://www.etch.com/
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the hospital-wide conversion ensures that all East Tennessee Children's patients will be cared for using the most technologically and clinically advanced noninvasive patient monitoring solutions available; risks related to our belief that Masimo Patient SafetyNet can help keep patients safer by noninvasively, continuously measuring and tracking their underlying physiological condition to help hospitals avoid preventable patient deaths and injuries associated with failure to rescue events, risks related to our assumptions of the repeatability of clinical results obtained, and risks related to the system's ability to significantly decrease traumatic critical events and costly ICU transfers to help improve patient outcomes and reduce costs; risks related to our belief that SpHb helps clinicians measure hemoglobin and trending noninvasively and continuously, and reduces the frequency of unnecessary blood transfusions; risks related to our believe Masimo SET virtually eliminates false alarms and increases a clinician's ability to detect life-threatening events; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Erica Estep
East Tennessee Children's Hospital
Phone: (865) 541-8276
Email: ESEstep@etch.com
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SedLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.

![]()

Holland Hospital Installs Masimo Noninvasive rainbow® Technology to Help Improve Patient Assessments and Speed Clinical Decisions
Use of SpHb® Noninvasive Hemoglobin Is Part of Leading Healthcare Facility's Standardization to Masimo SET® Pulse Oximetry
Holland, Michigan & Irvine, California – March 27, 2013 – Masimo (NASDAQ: MASI) announces that Holland Hospital, ranked as one of America's 100 Best Hospitals in 2013 by Healthgrades, is adding new clinical capabilities that allow clinicians to noninvasively and continuously monitor hemoglobin blood levels using Masimo SpHb®.
Holland Hospital is now leveraging Masimo technology with noninvasive hemoglobin (SpHb) – clinically shown to reduce risky and unnecessary blood transfusions, and speed up necessary blood transfusions, while dramatically reducing costs – in its operating rooms.
"Our anesthesiologists started using Masimo Pulse CO-Oximetry technology for noninvasive hemoglobin monitoring during surgeries that took a long time and had the potential for a lot of blood loss, such as spinal surgeries," said Jeffrey Hodges, manager of the Cardiopulmonary Department at Holland Hospital. "Based on those results, the anesthesiologists then requested that the use of Masimo pulse oximeters be expanded to other floors."
Hemoglobin levels are used as a primary indicator for red blood cell (RBC) transfusion, but conventional laboratory measurements are only available intermittently and results can be delayed in the period between blood draw and laboratory analysis. This time gap of information can lead to sub-optimal transfusion decisions.1 RBC transfusion overuse as well as delayed RBC transfusions can increase patient risk and cost of care. Multiple observational studies have shown that patients receiving RBC transfusions have an 88% higher mortality, 69% higher infection rate, and 250% higher rate of acute respiratory distress syndrome.2
SpHb monitoring provides real-time directional trends in hemoglobin, such as indicating stable hemoglobin when it may be perceived to be dropping, and rising hemoglobin when it may be perceived to not be rising fast enough. A recent award-winning study from Cairo University in Egypt also showed that once clinicians determined a transfusion was needed, they were able to initiate transfusions 82% faster – in about 9 minutes, compared to about 50 minutes for patients not being monitored by SpHb.3 That same study also showed SpHb can help clinicians reduce unnecessary and risky RBC transfusions, which can improve patient outcomes while dramatically lowering the cost of care.
The use of SpHb is part of Holland Hospital's standardization to Masimo SET® Measure-through Motion and Low Perfusion™ ™ pulse oximetry, shown to virtually eliminate false alarms4 and help clinicians better detect life-threatening events.5
"Like many health organizations, we're concerned about the safety of post-op patients on opioids, who are prone to respiratory depression," Hodges added. "With that in mind, we're making Masimo pulse oximetry a part of our standard of care."
Joe Kiani, founder and CEO of Masimo, stated: "We are honored that Holland Hospital, one of the top-performing hospitals in the nation with an inspiring reputation for excellence, has selected Masimo for its patient monitoring needs. As a forward-thinking healthcare organization that embraces best-in-class medical technologies, we are eager to help Holland Hospital improve its already superb patient safety and outcomes."
1 Friedman MT et al. Arch Pathol Lab Med. 2006 Apr;130(4):474-9.
2 Marik PE.et.al. Crit Care Med. 2008;36(9):2667-74
3 Wael NA, Maher F. Reduction in Red Blood Cell Transfusions during Neurosurgery with Noninvasive and Continuous Hemoglobin Monitoring. Proceedings of the Society for Technology in Anesthesia Annual Meeting ; 2013 Jan 9-12; Phoenix AZ.
4 Shah N, Ragaswamy H, Govindugari K, Estanol L. "Performance of three new-generation pulse oximeters during motion and low perfusion in volunteers." Journal of Clinical Anesthesia. 2012 (10.1016/j.jclinane.2011.10.012) https://www.sciencedirect.com
5 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2 Impact-of-Pulse-Oximetry-Surveillance-on-Rescue..
About Holland Hospital
Holland Hospital serves the health care needs of the greater Holland area and surrounding communities in Ottawa and Allegan counties with a 189-bed main campus and numerous off-site locations. The hospital medical staff includes more than 300 primary care and specialty physicians with support from 2,000 hospital employees. Holland Hospital is ranked among the nation's 100 Top Hospitals by Truven Health (Thomson Reuters) and Healthgrades, and has received ISO 9001:2008 certification by DNV Healthcare. More information about the Truven Health 100 Top Hospitals 2013 award can be found online to 100tophospitals@truvenhealth.com. Learn more about Holland Hospital's services and programs at hollandhospital.org
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-through Motion and Low Perfusion™ pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including total hemoglobin (SpHb®), contribute to positive clinical outcomes and patient safety in all patients and in every clinical situation; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including immediate and continuous results that enable earlier treatment without causing invasive trauma; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Tim Breed
Holland Hospital
Phone: (616) 394-3590
Email: tbreed@hollandhospital.org
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.



Hospital General Universitario Santa Lucia Is First in Spain to Install Masimo Patient SafetyNet™ System for Improved Oversight of its Neonatal Care Unit
Cartagena, Spain & Irvine, California – March 20, 2013 – Hospital General Universitario Santa Lucia, and Masimo (NASDAQ: MASI) today announced that the hospital has become the first in Spain to deploy Masimo Patient SafetyNet™, clinically shown to help improve patient outcomes and reduce costs.1
General Universitario Santa Lucia installed the Patient SafetyNet system to provide advanced monitoring of its most vulnerable patients in the neonatal basic care unit. Patient SafetyNet can help ensure patient safety by noninvasively and continuously measuring a patient's physiological conditions and detecting changes or abnormalities that signal declining health status in real-time. When changes occur in the measured values, which may indicate deterioration in the patient's condition, the system automatically sends wireless alerts directly to clinicians – prompting a potentially lifesaving response to the patient's bedside.
"The Patient SafetyNet implementation has enabled us to develop a process of admission for newborns that allows them to stay with their parents in a rooming-in basis, offering the maximum safety for these especially vulnerable patients," said Dr. Jose Luis Leante Castellanos, head of Santa Lucia's Neonatology Unit. "The staff has been extremely satisfied with the system."
The Masimo Patient SafetyNet system consists of Masimo SET®Measure-Through Motion and Low Perfusion™ pulse oximetry, with choice of patient-tolerant and easy-to-use ventilation monitoring with rainbow®Acoustic Monitoring or standard capnography, and remote monitoring and notification to help keep clinicians connected to patients. Patient SafetyNet has been clinically shown to help reduce rapid response activations, intensive care unit (ICU) transfers, and deaths related to opioid-induced respiratory depression.1
Masimo founder and CEO Joe Kiani stated: "We are truly proud and honored that General Universitario Santa Lucia, one of the most modern healthcare facilities in Spain, has selected Masimo technology to help protect patients and improve patient outcomes. Parents can be confident that with the Masimo Patient SafetyNet system, their little loved ones are being safely monitored at the bedside even when clinical staff is not in the room."
1 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. Available online
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET®outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that all neonatal patients at General Universitario Santa Lucia will be cared for using the most technologically and clinically-advanced noninvasive patient monitoring solutions available; risks related to our belief that Masimo Patient SafetyNet can help keep patients safer by noninvasively, continuously measuring and tracking their underlying physiological condition to help hospitals avoid preventable patient deaths and injuries associated with failure to rescue events, risks related to our assumptions of the repeatability of clinical results obtained, and risks related to the system's ability to significantly decrease traumatic critical events and costly ICU transfers to help improve patient outcomes and reduce costs; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Ana Ma Meseguer Barrionuevo
General Universitario Santa Lucia
Phone: 34 618 68 10 01
Email: anam.meseguer@carm.es
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SedLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


Masimo to Present at 25th Annual ROTH Conference
IRVINE, Calif., March 7, 2013 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the 25th Annual ROTH Conference at the Ritz-Carlton in Laguna Niguel, Calif. on Tuesday, March 19, 2013, at 10:30 a.m. Pacific Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. And in 2012, Masimo acquired the assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET®and Masimo rainbow®SET®technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Contact:
Sheree Aronson
(949) 297-7043
saronson@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.



University of Virginia Medical Center Installs Masimo Noninvasive rainbow® Technologies to Improve Patient Assessments and Speed Clinical Decisions
Leading Healthcare Facility Standardizes to Masimo Noninvasive Hemoglobin (SpHb®) and Acoustic Respiration Rate (RRa™) Technologies in Key Care Areas
Charlottesville, Virginia & Irvine, California - February 20, 2013 - Masimo (NASDAQ: MASI) announces that the University of Virginia Medical Center has added new clinical capabilities that allow clinicians to noninvasively and continuously monitor hemoglobin blood levels using Masimo SpHb® and respiration rate using Masimo RRa™.
UVA Medical Center now leverages Masimo Radical-7® Pulse CO-Oximeters with noninvasive hemoglobin (SpHb®) - clinically shown to reduce risky and unnecessary blood transfusions while dramatically reducing costs1 - in its operating rooms, obstetric, and surgical intensive care units.
"We use the Radical-7 with SpHb primarily for complex spine cases, where we have a particular need to track hemoglobin levels on a continuous basis," said Dr. Marcel Durieux, MD, of UVA Medical Center's Department of Anesthesiology. "Having a trending measure rather than having to rely only on intermittent blood gas measurement is helpful, and it allows us to better time when to get a blood gas."
Hemoglobin levels are used as a primary indicator for red blood cell (RBC) transfusion, but laboratory measurements are only available intermittently and results can be delayed in the period between blood draw and laboratory analysis. This time gap of information can lead to sub-optimal transfusion decisions.2 Because SpHb monitoring provides real-time directional trends in hemoglobin - such as indicating stable hemoglobin when it may be perceived to be dropping, and rising hemoglobin when it may be perceived to not be rising fast enough - it can help clinicians initiate necessary transfusions faster. A recent study from Cairo University in Egypt showed that once clinicians determined a transfusion was needed, they were able to initiate transfusions 82% faster - in about 9 minutes, compared to about 50 minutes for patients not being monitored by SpHb.1
That same study also showed SpHb can reduce unnecessary and risky RBC transfusions, which can improve patient outcomes while lowering the cost of care.1 RBC transfusion is one of the most frequent procedures performed in U.S. hospitals, with one in 10 patients receiving one or more blood units. 3 RBC transfusion overuse can increase patient risk and cost of care. Multiple observational studies have shown that patients receiving RBC transfusions have an 88% higher mortality rate, 69% higher infection rate, and 250% higher rate of acute respiratory distress syndrome.4
While some clinicians are concerned about withholding RBC transfusions, multiple randomized controlled trials indicate that restrictive transfusion practices - those in which significantly lower hemoglobin triggers are used to determine need for transfusion - are safe.5 In addition, the cost of each RBC unit is estimated between $522 and $1,183 per unit, without including morbidity-associated costs.6
In its post-anesthesia care areas, UVA Medical Center also will be leveraging Masimo Acoustic Respiration Rate (RRa™) - enabling clinicians to noninvasively and continuously assess patient breathing to facilitate earlier detection of respiratory compromise and patient distress. Featuring an innovative adhesive sensor with an integrated acoustic transducer that is easily and comfortably applied to the patient's neck, RRa helps to meet APSF guidelines for monitoring post-operative patients.7
"UVA Medical Center enjoys a well-earned reputation for providing quality patient care," said Joe Kiani, CEO and founder of Masimo. "We at Masimo are honored to have been a partner with UVA for many years, and we're committed to continuing to provide the advanced, high-quality medical technology this leading healthcare organization needs to help further improve patient outcomes and reduce costs."
###
1 Wael NA, Maher F. Reduction in Red Blood Cell Transfusions during Neurosurgery with Noninvasive and Continuous Hemoglobin Monitoring. Proceedings of the Society for Technology in Anesthesia Annual Meeting ; 2013 Jan 9-12; Phoenix AZ. Available http://www.masimo.com/pdf/clinical/hemoglobin/Wael-STA-2013.pdf.
2 Friedman MT et al. Arch Pathol Lab Med. 2006 Apr;130(4):474-9.
3 AHRQ. Inpatient Sample. 1997-2007.
4 Marik PE.et.al. Crit Care Med. 2008;36(9):2667-74
5 Carson et al. Cochrane Database Syst Rev. 2012 Apr 18;4:CD002042.
6 Shander A et al. Transfusion. 2010;50(4):753-765.
7 Weinger MB. Dangers of postoperative opioids: APSF workshop and white paper address prevention of postoperative respiratory complications; APSF Newsletter. 2006; 21(4): 61-88.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOCTM), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI ®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic MonitoringTM, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SedLine®, a pioneer in the development of innovative brain function monitoring technology and devices. In 2012, Masimo acquired assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and acquired Phasein AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow ® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including total hemoglobin (SpHb®) and acoustic respiration rate (RRa™), contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including immediate and continuous results that enable earlier treatment without causing invasive trauma; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Eric Swensen
UVA Medical Center
Phone: (434) 924-5770
Email: ews3j@virginia.edu
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


Masimo to Present at Raymond James 34th Annual Institutional Investors Conference
IRVINE, Calif., February 20, 2013 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the Raymond James 34th Annual Institutional Investors Conference at the JW Marriott Orlando Grande Lakes Hotel in Orlando, Fla. on Wednesday, March 6, 2013, at 9:50 a.m. Eastern Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. And in 2012, Masimo acquired the assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET®and Masimo rainbow®SET®technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Contact:
Sheree Aronson
(949) 297-7043
saronson@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Continues Its Advocacy on Behalf of Newborns During Congenital Heart Defects (CHD) Awareness Week Although Pulse Oximetry Screening Gains Ground, Most Infants Go Unscreened in the U.S.
Irvine, California – February 15, 2013 – Congenital Heart Defects (CHD) are the leading cause of infant deaths – affecting about 1 in 125 babies.1,2 In the U.S., one in three infants with a potentially life-threatening CHD leaves the hospital undiagnosed.3
Early detection through pulse oximetry screening and intervention are keys to saving the lives of babies with CHDs.4 The screening determines the amount of oxygen in the blood and pulse rate. It is inexpensive, noninvasive, and fast. As part of Congenital Heart Defect Awareness Week (Feb. 7-14), Masimo (NASDAQ: MASI) continued to advocate for pulse oximetry screening of all newborns by demonstrating proper sensor placement during the America Heart Association's "Heart on the Hill Day" in St. Paul, Minn., earlier this week. Minnesota is joining a growing number of states that are enacting or have enacted legislation that require screening newborns for Critical Congenital Heart Disease (CCHD) prior to discharge from hospitals. To date, California, Connecticut, Delaware, Indiana, Maryland, New Hampshire, New Jersey, Tennessee and West Virginia have enacted such legislation, according to the Newborn Coalition.
Although screening newborns with Measure-Through Motion and Low Perfusion Masimo SET® pulse oximetry has been shown to increase CCHD detection by 34%,4 CCHD screening is not currently included in most state newborn screening panels, according to the Centers for Disease Control and Prevention.
Recognizing the growing need for universal CCHD screening and the "emerging evidence base for the utility of early diagnosis and detection of CCHD via measurement of blood oxygen saturation," the U.S. Department of Health and Human Services (HHS) made CCHD screening by pulse oximetry a nationwide standard by adding it to the Recommended Uniform Screening Panel (RUSP) Guidelines in 2011. Federal guidelines recommend pulse oximetry screening with "motion-tolerant pulse oximeters" that "have been validated in low perfusion conditions."5
To effectively screen for CCHD, using the right technology matters. Last year Masimo received FDA 510(k) clearance for SET® pulse oximeters, rainbow® Pulse CO-Oximeters®, and neonatal sensors with labeling for screening newborns for CCHD. This marked the first time the FDA cleared specific labeling indicating the use of pulse oximeters, in conjunction with a physical exam, to screen newborns for CCHD.
Validation of SET®'s advantage for CCHD screening is provided in a 2005 study by Dr. Anne de Wahl Granelli that evaluated the sensitivity of various SpO2 cut points for CCHD screening. In the study, Dr. Granelli evaluated a SET® and non-SET pulse oximeter which has been referred to as "next generation" technology (GE Ohmeda Tuffsat).6 The "next generation" technology resulted in 41% of post-ductal SpO2 values below 95%, while the SET® pulse oximeter recorded only 1%.7 The high number of false positives with non-SET pulse oximetry led Dr. Granelli to abandon use of it and focus exclusively on signal extraction technology with SET® pulse oximeters for future CCHD studies. This result is not surprising, given that CCHD screening is often performed during motion and low perfusion.
To ensure that hospitals have the right technology in place to perform CCHD screening on newborns, Masimo announced the HEART Program (Help Ensure Access to the Right Technology). It enables hospitals in countries where Masimo has a presence that want to perform CCHD screening with a Masimo SET® pulse oximeter, but do not have one and do not have funds to purchase one, to receive a free Masimo SET® pulse oximeter. More details are available at https://www.masimo.com/heartprogram. Offering the HEART program internationally provides an opportunity to help solve the global CCHD burden around the world as health officials from the United Arab Emirates (UAE) to Beijing (China) and the UK have embraced Masimo pulse oximetry screening of newborns for CCHD.
"Early on Masimo SET® technology, because of its ability to measure-through motion, was an instrumental part of the protocol that dramatically reduced neonatal eye damage and Retinopathy of Prematurity in countless newborns," said Joe Kiani, founder and CEO of Masimo. "Now we are seeing Masimo technology play an increasing role in the early detection of CCHD in newborns, which is helping save precious lives and spare families the soul-crushing pain of losing an infant. For Masimo, every week is CHD Awareness Week."
To download the Action Plan to Address Failure to Detect Critical Congenital Heart Disease (CCHD) presented at this year's Patient Safety, Science & Technology Summit, please visit: http://www.patientsafetysummit.org/challenges/challenge-6-cchd-screening-in-newborn-infants.aspx
Also available online, Masimo's Newborn CCHD Screening Step-by-Step Instructional Guide, which provides another helpful resource to clinicians and hospitals looking to implement CCHD screening in their institutions. Visit: http://www.masimo.com/heartprogram/documents/LAB6252B_Sales_Tool_CCHD_Screening_Adhesive_Sensor.pdf
# # #
1 National Heart, Lung and Blood Institute. Congenital Heart Defects. December 2007.
2 Congenital Cardiovascular Defects: Current Knowledge: A Scientific Statement From the American Heart Association Council on Cardiovascular Disease in the Young. Circulation, volume 115, June 12, 2007, pages 2995-3014.
3 Wren C, Reinhardt Z, Khawaja K. Twenty-year trends in diagnosis of life-threatening neonatal cardiovascular malformations. Arch Dis Child Fetal Neonatal Ed. 2008;93:F33–F35.
4 Andrew K Ewer, Lee J Middleton, Alexandra T Furmston, Abhay Bhoyar, Jane P Daniels, Shakila Thangaratinam, Jonathan J Deeks, Khalid S Khan. "Pulse oximetry screening for congenital heart defects in newborn infants (PulseOx): a test accuracy study." The Lancet 2011: Vol. 378; No. 9793; pp 785-794. Available http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(11)60753-8/fulltext#article_upsell.
5 Secretary of Health & Human Services letter to the Secretary's Advisory Committee on Heritable Disorders in Newborns and Children (SACHDNC); dated September 21, 2011. Available http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/recommendations/correspondence/cyanoticheartsecre09212011.pdf .
6 http://www.gehealthcare.com/euen/patient_monitoring/products/imm-monitoring/pulse-oximeters/index.html
7 de Wahl Granelli A et al. Acta Paediatr. 2005 Nov;94(11):1590-1596.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOCTM), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SedLine®, a pioneer in the development of innovative brain function monitoring technology and devices. In 2012, Masimo acquired assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and acquired Phasein AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo SET improves CHD detection in newborns before hospital discharge, risks related to our assumptions of the repeatability of clinical results obtained, and risks related to our assumptions that Masimo SET pulse oximetry technology is a superior solution for CHD detection and newborn screening applications, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo
Phone: (949) 297-7434
Email: mdrummond@masimo.com


Masimo Announces Adoption of Stock Repurchase Program
Irvine, California – February 14, 2013 – Masimo (NASDAQ: MASI) today announced that its Board of Directors has authorized the repurchase of up to 6 million shares of the company's common stock. The stock repurchase program may be carried out at the direction of the company through open market purchases, block trades, one or more trading plans adopted in accordance with Rule 10b5-1 of the Securities and Exchange Commission, and in privately negotiated transactions. The repurchase program will become effective on February 20, 2013 and is expected to continue for a period of up to 36 months unless it is terminated earlier by the Board of Directors. Any repurchases will be subject to the availability of stock, general market conditions, the trading price of the stock, available capital, alternative uses for capital and the company's financial performance. The company expects to fund the stock repurchase program through its available cash, future cash from operations, or other potential sources of capital.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "In our five years as a public company, Masimo has established a solid track record of returning value to stockholders and this latest stock repurchase program further underscores our continued belief and optimism in Masimo's long-term growth prospects. Our management team and Board of Directors are confident in the power of our technology platform, global franchise and business model to take full advantage of our growth potential, and we believe the stock represents an attractive value relative to our future outlook."
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SedLine®, a pioneer in the development of innovative brain function monitoring technology and devices. In 2012, Masimo acquired assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and Phasein AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET®and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ...by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements, including statements that the company will generate future cash flow, the repurchase of company stock constitutes an opportunity to increase stockholder value, available cash should provide sufficient liquidity to support the stock repurchase program, and the repurchase of our stock represents an attractive investment. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the integration of acquisitions; the amount and type of equity awards that we may grant to employees and service providers in the future; generating sufficient capital from operations, or obtaining sufficient capital from other sources, to repurchase our shares of common stock pursuant to the stock repurchase program; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws. The repurchase program authorization does not require the company to purchase a specific number of shares and the program may be modified, suspended or terminated at any time. The timing and number of shares repurchased, if any, pursuant to the stock repurchase authorization will be subject to a number of factors, including current market conditions, legal constraints and available cash or other sources of funding. This press release is not an offer to purchase any securities.
Investor Contact:
Sheree Aronson
(949) 297-7043
saronson@masimo.com
Media Contact:
Mike Drummond
(949) 297-7434
mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo Reports Fourth Quarter and Full Year 2012 Financial Results; Provides 2013 Financial Guidance
Q4 2012 Highlights (compared to Q4 2011):
- Product revenue rose 20% to $125.3 million
- Masimo rainbow revenue rose 13% to $11.1 million
- Shipped 42,700 Masimo SET®and Masimo rainbow®SET units
- Net income was $15.0 million, with EPS of $0.26 versus $0.23 in the year-ago period
Full Year 2012 Highlights (compared to 2011):
- Product revenue rose 14% to $464.9 million
- Masimo rainbow revenue rose 18% to $40.3 million
- Shipped 146,400 Masimo SET®and Masimo rainbow®SET units, increasing worldwide installed base by 11%
- Net income was $62.3 million, with EPS of $1.07 versus $1.05 in 2011
Irvine, California, February 14, 2013 – Masimo (NASDAQ: MASI) today announced its financial results for the fourth quarter and year ended December 29, 2012.
Masimo's total fourth quarter revenue, including royalties, rose 18% to $132.2 million, compared to $112.3 million for the fourth quarter of 2011. Fourth quarter 2012 product revenue rose 20% to $125.3 million, compared to $104.7 million for the fourth quarter of 2011. The company's worldwide end-user product revenue grew 18% in the fourth quarter of 2012 and represented 85% of product revenue. OEM sales, which accounted for 15% of product revenue, rose 28% compared to the same period in 2011. Revenue from sales of Masimo rainbow products rose 13% to $11.1 million in the fourth quarter, compared to $9.8 million for the fourth quarter of 2011.
Net income for the fourth quarter of 2012 was $15.0 million, or $0.26 per diluted share, compared to net income of $13.8 million, or $0.23 per diluted share, in the fourth quarter of 2011. The fourth quarter 2012 results were impacted by a $1.3 million non-operating expense primarily related to realized and unrealized net losses on foreign currency denominated transactions due to strengthening of the U.S. dollar against the Japanese yen, and suspension of the federal research tax credit in fiscal 2012, which combined to reduce fourth quarter 2012 EPS by approximately $0.03.
For 2012, Masimo's total revenue, including royalties, rose 12% to $493.2 million, compared to $439.0 million for 2011. Total 2012 product revenue rose 14% to $464.9 million, compared to $406.5 million for 2011. The company's worldwide end-user revenue grew 16% in 2012 and represented 85% of product revenue. OEM sales, which accounted for 15% of product revenue, rose 8% compared to 2011. Revenue from sales of Masimo rainbow products rose 18% to $40.3 million in 2012, compared to $34.1 million in 2011. Total 2012 rainbow revenue included a 48% increase in total hemoglobin (SpHb) sales compared to 2011.
Net income for 2012 was $62.3 million, or $1.07 per diluted share, compared to net income of $63.7 million, or $1.05 per diluted share, in 2011. The 2012 results were impacted by a $1.4 million non-operating expense primarily related to realized and unrealized net losses on foreign currency denominated transactions due to strengthening of the U.S. dollar against the Japanese yen, and the suspension of the federal research tax credit in fiscal 2012, which combined to reduce 2012 EPS by approximately $0.03. The 2012 results also included a $0.06 per share loss attributed to Masimo's 2012 acquisitions of PHASEIN AB and Spire Semiconductor.
During the fourth quarter, the company shipped approximately 42,700 Masimo SET pulse oximetry and Masimo rainbow SET Pulse CO-Oximetry units, excluding handheld units, up 24% compared to approximately 34,400 in the same prior-year period. For 2012, Masimo shipped approximately 146,400 Masimo SET pulse oximetry and Masimo rainbow SET Pulse CO-Oximetry units, excluding handheld units, down 1% compared to approximately 148,200 in 2011. Masimo estimates its worldwide installed base as of December 29, 2012 to be 1,088,000 units, up 11% from 979,000 units as of December 31, 2011.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "We finished 2012 with a 20% rise in fourth quarter product revenue. We've entered 2013 fully focused on advancing our mission, strategy, and 2013 objectives, which is to continue to grow our core SET Pulse Oximetry business by increasing our presence in critical care and the general ward, while leveraging our breakthrough rainbow Pulse CO-Oximetry platform to pursue new opportunities in and beyond the hospital setting."
As of December 29, 2012, Masimo's cash and cash equivalents were $71.5 million, compared to $129.9 million as of December 31, 2011. The change reflects primarily net cash generated from operations, offset by $37.4 million in cash used to acquire PHASEIN AB and Spire Semiconductor, $26.3 million in cash used to repurchase shares of Masimo common stock and $57.3 million used to pay a special $1.00 per share cash dividend to stockholders on December 11, 2012.
2013 Financial Guidance
Masimo expects fiscal 2013 total revenue to be approximately $548 million, including product revenue of $520 million and royalty revenue of $28 million. Included in the 2013 product revenue guidance is a rainbow revenue expectation of $50 million. The company expects fiscal 2013 GAAP earnings per share to be $1.14. Each of the components of Masimo's guidance set forth above is an estimate only and actual performance could differ.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at https://www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 92598385. After the live webcast, the call will be available on Masimo's website through March 14, 2013. In addition, a telephonic replay of the call will be available through February 28, 2013. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 92598385.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. And in 2012, Masimo acquired the assets of Spire Semiconductor, LLC, a maker of advanced light emitting diode (LED) and other advanced component-level technologies; and PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ...by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies; demand for our technologies; and expectations for total revenue, royalty revenue and product revenue, including rainbow revenue, and GAAP earnings per share, for the full fiscal year 2013, as well as the expected benefit from the recent reinstatement of the federal research tax credit . These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the integration of acquisitions; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact:
Sheree Aronson
(949) 297-7043
saronson@masimo.com
Media Contact:
Mike Drummond
(949) 297-7434
mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.
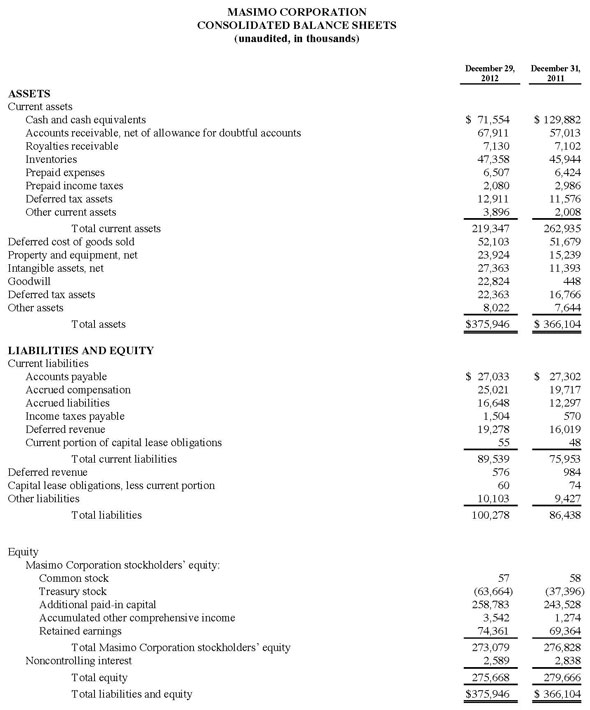
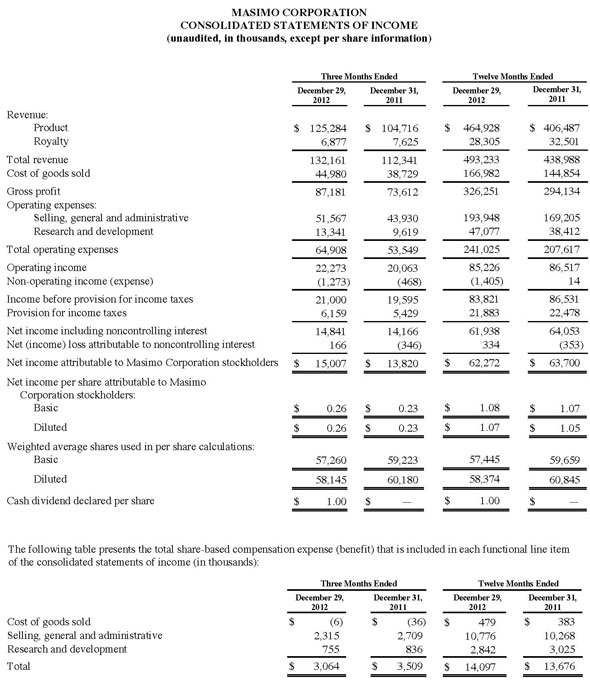
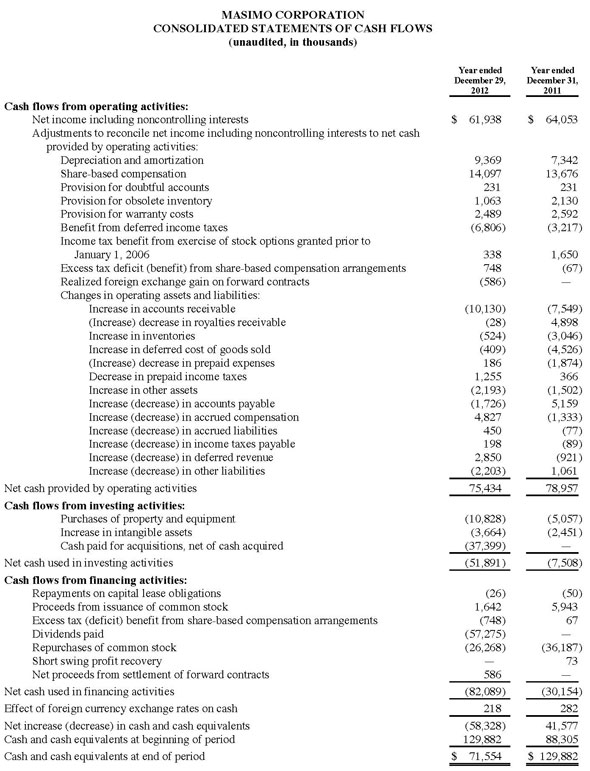


Libya Becomes First to Standardize Its Blood Donation Centers to Masimo Pronto-7® for Quick, Noninvasive Spot-Checking of Hemoglobin(SpHb®), SpO2, Pulse Rate, and Perfusion Index
Masimo SpHb® Now the National Standard of Care at Blood Donation Centers and Operating Rooms Throughout Libya
Dubai, UAE & Irvine, California – January 31, 2013 – Masimo (NASDAQ: MASI) announced today that Libya has become the first country to begin using the Masimo Pronto-7®— a handheld noninvasive hemoglobin spot-check device — to screen potential blood donors for low hemoglobin level in all of its major blood-donation centers. The announcement comes at Arab Health, the world's longest-running healthcare exhibition and congress held each January in Dubai.
 Donating blood prior to your own surgical procedure or for those of friends and family members is common practice throughout the Middle East. However, until the use of Pronto-7, centers in Libya were using invasive blood draws to test donors for low blood count or anemia. It is estimated that about 10% of people who attempt to donate blood are deferred because of a low hemoglobin level.1
Donating blood prior to your own surgical procedure or for those of friends and family members is common practice throughout the Middle East. However, until the use of Pronto-7, centers in Libya were using invasive blood draws to test donors for low blood count or anemia. It is estimated that about 10% of people who attempt to donate blood are deferred because of a low hemoglobin level.1
The palm-sized Pronto-7, with dimensions of just 13 cm x 7.2 cm x 2.5 cm (5.1" x 2.8" x 1") and weight of 296 grams (10.5 ounces), offers a breakthrough solution for quick and easy spot-check measurements of hemoglobin, SpO2, pulse rate, and perfusion index in less than one minute —without needles, time-consuming laboratory analysis, blood contamination, hazardous medical waste, and patient discomfort associated with traditional blood tests.
"The Pronto-7's accuracy, portability and ease-of-use helps clinicians protect donors from unintentional harm," said Dr. Nurideen Abdulhamid Dagman, Libya Minister of Health and former Director of the Benghazi Central Blood Bank. "With the use of advanced technologies such as the Pronto-7, Libya is demonstrating its commitment to patient safety."
Standardizing blood donor assessments using the Pronto-7 follows Libya's national adoption of Masimo continuous noninvasive hemoglobin (SpHb®) as the standard of care in the country's operating rooms in 2008.
"Libya's leadership in implementing Masimo's noninvasive hemoglobin monitoring technology as a national standard of care, first in operating rooms and now at blood donation centers, shows the country's commitment to adopting advanced medical technologies that improve patient care and safety," said Masimo founder and CEO Joe Kiani. "We applaud their efforts to put patient safety first and acknowledge the tireless work of Motahida Medical Co., our distributor in Libya, for making this achievement possible. Masimo is dedicated to expanding our presence in the Middle East and helping this very important region to continue to adopt leading-edge technologies for the betterment of patient care and safety."
1 BloodCenter of Wisconsin. Donating Blood, Investigators: Alan E. Mast, M.D., Ph.D. Link. Accessed: February 2, 2009.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SedLine®, a pioneer in the development of innovative brain function monitoring technology and devices. In 2012, Masimo acquired assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and Phasein AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors including: risks related to our assumptions regarding the repeatability of clinical results, risks related to our assumptions that Masimo SpHb accurately tracks and trends Hb changes in all patients, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo to Report Fourth Quarter and Full Year 2012 Financial Results after Market Close on Thursday, February 14
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., January 30, 2013 -- Masimo (NASDAQ: MASI) announced today that it will release fourth quarter and full year 2012 financial results for the period ended December 29, 2012, after the market closes on Thursday, February 14, 2013. The conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at https://www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 92598385. After the live webcast, the call will be available on Masimo's website through March 14, 2013. In addition, a telephonic replay of the call will be available through February 28, 2013. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 92598385.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. And in 2012, Masimo acquired the assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Contact:
Sheree Aronson
(949) 297-7043
saronson@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.



Study Featuring Transfusion Impact of Masimo SpHb® Monitoring Receives 2013 Best Clinical Application of Technology Award at Society for Technology in Anesthesia Annual Meeting
Irvine, California – January 11, 2013 – A new study using Masimo (NASDAQ: MASI) noninvasive and continuous total hemoglobin (SpHb) monitoring technology has received the 2013 Best Clinical Application of Technology Award at the Society for Technology in Anesthesia (STA) Annual Meeting.
The winning study, "Reduction in Red Blood Cell Transfusions during Neurosurgery with Noninvasive and Continuous Hemoglobin Monitoring" evaluated the use of SpHb monitoring in patients scheduled for neurosurgery and found that SpHb monitoring helped clinicians achieve a 56% reduction in the frequency of multi-unit RBC transfusions and a 47% reduction in average number of RBC units transfused. Moreover, once clinicians determined a transfusion was needed, they were able to initiate transfusions 82% faster using SpHb monitoring.1
Dr. Wael Awada stated: "We are so proud to receive the STA award for best clinical application of technology. Our study shows that Masimo's SpHb monitoring improves transfusion decision making and results in dramatic cost savings for hospitals."
"Studies show that blood transfusions increase mortality, morbidity, and healthcare costs. With this backdrop, the importance of Masimo SpHb monitoring to help reduce the frequency and quantity of blood transfusions, while reducing associated costs cannot be underestimated. We thank Dr. Awada and his colleagues for their contribution to medicine with this important clinical study and congratulate them for this prestigious award," stated Dr. Michael O'Reilly, Chief Medical Officer at Masimo.
1 Wael NA, Maher F. Reduction in Red Blood Cell Transfusions during Neurosurgery with Noninvasive and Continuous Hemoglobin Monitoring. Proceedings of the Society for Technology in Anesthesia Annual Meeting ; 2013 Jan 9-12; Phoenix AZ. Available http://www.masimo.com/pdf/clinical/hemoglobin/Wael-STA-2013.pdf.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including total hemoglobin (SpHb), contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
(949) 297-7434
mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


New Study Shows Masimo Noninvasive & Continuous Total Hemoglobin (SpHb®) Monitoring Significantly Reduces Blood Transfusions and Costs in High Blood Loss Surgery
Average Reduction of 0.9 Red Blood Cell Units per Patient Represents $470 to $1,065 in Cost Savings per Patient
Irvine, California – January 10, 2013 – Noninvasive and continuous total hemoglobin (SpHb) monitoring technology from Masimo (NASDAQ: MASI) helps clinicians reduce intra-operative red blood cell (RBC) transfusions during high blood-loss surgery, speeds time to transfusion when indicated, and saves significant costs, according to a new study unveiled at the Society for Technology in Anesthesia (STA) annual meeting.1 The study was one of just five receiving the distinguished 2013 award for the best research at the STA meeting.
RBC transfusion is one of the most frequent procedures performed in U.S. hospitals, with one in ten inpatients receiving one or more blood units.2 While blood loss during surgery is a known risk factor, RBC transfusion overuse can increase patient risk and cost of care. Meta-analysis of pooled results from multiple observational studies, each of which adjusts for risks between patients, shows patients receiving RBC transfusions have an 88% higher mortality, 69% higher infection rate, and 250% higher rate of ARDS.3 While some clinicians are concerned with the idea of withholding RBC transfusions, multiple randomized controlled trials indicate that restrictive transfusion practices – those in which significantly lower hemoglobin triggers are used to determine need for transfusion – are safe.4 In addition, the cost of each RBC unit is estimated between $522 and $1,183 per unit, without including morbidity-associated costs.5
Hemoglobin levels are used as a primary indicator for RBC transfusion, but laboratory measurements are only available intermittently and results can be delayed in the period between blood draw and laboratory analysis. This time gap of information can lead to sub-optimal transfusion decisions.6 Given the risks and costs of RBC transfusions, there is a growing recognition of the need to implement strategies to reduce transfusions. The American Medical Association and The Joint Commission recently identified RBC transfusions as one of the top five overused procedures in medicine,7 and The Joint Commission has introduced Patient Blood Management Measures that encourage hospitals to evaluate appropriateness of transfusions as a continuous quality indicator.8 A previous study conducted at Massachusetts General Hospital during orthopedic surgery (a low blood loss setting) showed that SpHb monitoring helped clinicians reduce RBC transfusion frequency (from 4.5% to 0.6% of patients, an 87% reduction) and average RBC units per patient (from 0.10 to 0.01 units per patient, a 90% reduction),9 but the current study is the first to report the impact of SpHb in high blood loss surgery.
Drs. N.A. Wael and Fawzy Mahmoued at Cairo University in Egypt conducted a prospective cohort study in patients scheduled for neurosurgery. One patient scheduled for neurosurgery the next day was randomly selected using the sealed envelope method to be screened for SpHb Group inclusion, and following enrollment into the SpHb Group, the other patients also scheduled for surgery on the same day were screened for inclusion into the Standard Care Group. The SpHb Group received typical anesthesia care plus SpHb monitoring (Masimo Radical-7®, v7748 & R2-25 adult rainbow®ReSposable sensor). The SpHb Group followed the same transfusion practice as the Standard Care Group except the anesthesiologist was guided by the addition of SpHb, with blood samples still taken before and after transfusion.
A total of 106 patients were enrolled – one group with SpHb monitoring and one group without SpHb monitoring. The SpHb Group had a 56% reduction in the frequency of multi-unit RBC transfusions (from 73% to 32% of patients receiving three or more units, p<0.01), and 47% reduction in average number of RBC units transfused (from 1.9 to 1.0 units per patient, p<0.001). Moreover, once clinicians determined a transfusion was needed, they were able to initiate transfusions 82% faster (in about 9 minutes, compared to about 50 minutes for those not being monitored by SpHb, p<0.001).
The researchers noted that noninvasive and continuous SpHb monitoring provides real-time directional trends in hemoglobin, such as indicating stable hemoglobin when it may be perceived to be dropping, and rising hemoglobin when it may be perceived to not be rising fast enough, thus changing transfusion decision making.
"Based on the average 0.9 RBC unit reduction per patient in the SpHb Group, SpHb monitoring could save $470 to $1,065 per patient monitored and $469,800 to $1,064,700 per 1,000 surgeries performed," the researchers said. They concluded: "SpHb monitoring reduced intra-operative RBC transfusions during high blood-loss surgery. Based on the RBC reduction shown with SpHb monitoring, hospitals could significantly reduce costs with this approach."
"Hospitals looking to increase quality and reduce costs are now starting to see RBC transfusion overuse as a major target," said Masimo founder and CEO Joe Kiani. "This high blood loss study from Cairo and the low blood loss study from Boston, show that SpHb can be a valuable tool in reducing unnecessary blood transfusion, and the Cairo study shows that for those who need transfusion, SpHb can speed up its delivery. This, along with the data that shows blood transfusion increases mortality and morbidity is why we launched our Blood Transfusion Related Cost Reduction (BTR-CR, "Better Care") Guarantee, which guarantees a hospital's blood-transfusion related cost reductions will be greater than the cost of SpHb monitoring – representing a win for all involved, especially the patients."
For more information about SpHb or BTR-CR, contact 888-44BTRCR or http://masimo.custhelp.com/ci/documents/detail/2/Better_Care_Guarantee
- 1 Wael NA, Maher F. Reduction in Red Blood Cell Transfusions during Neurosurgery with Noninvasive and Continuous Hemoglobin Monitoring. Proceedings of the Society for Technology in Anesthesia Annual Meeting ; 2013 Jan 9-12; Phoenix AZ. Available http://www.masimo.com/pdf/clinical/hemoglobin/Wael-STA-2013.pdf.
- 2 AHRQ. Inpatient Sample. 1997-2007.
- 3 Marik PE.et.al. Crit Care Med. 2008;36(9):2667-74
- 4 Carson et al. Cochrane Database Syst Rev. 2012 Apr 18;4:CD002042.
- 5 Shander A et al. Transfusion. 2010;50(4):753-765.
- 6 Friedman MT et al. Arch Pathol Lab Med. 2006 Apr;130(4):474-9.
- 7 Joint Commission Perspectives. The Joint Commission Continues to Study Overuse Issues. Volume 32, Number 5, 2012: 4-8(5).
- 8 https://www.jointcommission.org/accreditation-and-certification/certification/certifications-by-setting/hospital-certifications/patient-blood-management-certification/
- 9 Ehrenfeld JM, Henneman JP, Sandberg WS. "Impact of Continuous and Noninvasive Hemoglobin Monitoring on Intraoperative Blood Transfusions." American Society Anesthesiologists. 2010;LB05
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including total hemoglobin (SpHb), contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
(949) 297-7434
mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo iSpO2™ Pulse Oximeter for iOS Platform (iPhone, iPad & iPod touch) for Consumers* to Debut at CES Visit Booth 26324 for a Chance to Win an iSpO2™ and iPad, iPad mini or iPod touch and Meet Guinness World Record Breath Holder Stig "The Ultimate Superhuman" Severinsen
Irvine, California – January 3, 2013 – Masimo (NASDAQ: MASI) makes its debut at CES in Las Vegas next week with iSpO2™, the pulse oximeter cable and sensor utilizing Masimo SET®technology for use with iPhone, iPad or iPod touch with 30-pin connector.
 |
| CAPTION: Masimo iSpO2™ pulse oximetry cable and sensor (available at iSpO2.com) is compatible with the iPhone, iPad, or iPod touch. |
A variety of people purchase pulse oximeters for personal uses, including fitness buffs and those going to high altitudes. Thousands of pulse oximeters, which measure blood oxygenation and pulse rate, are sold to consumers each year.
The Masimo iSpO2™ is a consumer pulse oximeter to noninvasively measure blood oxygenation, pulse rate and perfusion index with the same technology used in leading hospitals worldwide, providing accurate measurements even during the challenging conditions of motion and low perfusion.
iSpO2™, available atiSpO2.com and Amazon, is easy to set up and use with your iPhone, iPad or iPod touch (30-pin connector):
• Connect the iSpO2™ cable to your iPhone, iPad or iPod touch
• Slip the iSpO2™ sensor on your ring finger
• Your results appear on screen
iSpO2™ app provides immediate access to your data history – allowing you to view measurements, graphs, and trending over time – with the ability to export data history into .CSV files for use with text editing and spreadsheet programs such as Microsoft Excel.
Helping showcase iSpO2™ at CES will be Stig "The Ultimate Superhuman" Severinsen, who in 2012 set the Guinness World Record for holding his breath – 22 minutes – and is a four-time world champion freediver with a doctorate in medicine. Severinsen, author of "breatheology – the art of conscious breathing," will be at Booth 26324 Jan. 9 to conduct breath-holding feats and demonstrate iSpO2™.
Visit Masimo at Booth 26324 for daily drawings Jan. 8-10 to be among the first to own iSpO2™. The drawings also include chances to win a new iPad, iPad mini or iPod touch with 30-pin connector.
"We made iSpO2™ for consumers interested in obtaining the accurate SpO2, pulse rate, and perfusion index readings that Masimo has been known for throughout the healthcare industry," said Masimo founder and CEO Joe Kiani. "At CES, consumers will have an opportunity to see for themselves the quality and utility of iSpO2™."
Please visit iSpO2.com, Amazon.com or the App Store for purchasing information.
* iSpO2™ is for short-term sports and aviation use and is not intended for medical use.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET®outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOCTM), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic MonitoringTM, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SedLine®, a pioneer in the development of innovative brain function monitoring technology and devices. In 2012, Masimo acquired assets of Spire Semiconductor, LLC, maker of advanced light emitting diode (LED) and other advanced component-level technologies; and Phasein AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at https://www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors related to the new iSpO2, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.
