Home / Newborn Care / CCHD Screening
CCHD Screening

CCHD Screening With SET® Pulse Oximetry
Clinically Proven Newborn Screening for Critical Congenital Heart Disease (CCHD) with Masimo SET® Pulse Oximetry
Now featuring simultaneous Dual SET Oximetry™ on one convenient display.
Why Screen for CCHD?
Why Screen for CCHD?
Traditionally, following birth, newborns were observed for evidence of congenital heart defects (CHDs) by physical assessment and monitoring for common symptoms.1 Today, studies show that these methods alone may fail to detect some infants with a Critical CHD (CCHD) before discharge.2,3 Adding screening with pulse oximetry can help better assess CCHD.4
Masimo SET® in CCHD Screening Studies
Masimo SET® in CCHD Screening Studies
There have been numerous large published CCHD screening studies that exclusively used Masimo SET® pulse oximeters and sensors, two of which (59,876 subjects) were the basis for a CCHD workgroup recommendation for screening protocols.3,7,15 Cumulatively, these studies represent over 300,000 infants2,3,5-14 and include the largest CCHD screening study to date, with over 122,738 subjects.2
Notable studies with a large number of subjects include:
- 10,009 infants: de-Wahl Granelli A et al. Acta Paediatr. 2007.
- 50,008 infants: Meberg A et al. J of Peds. 2008.
- 39,821 infants: de-Wahl Granelli A et al. BMJ. 2009.
- 20,055 infants: Ewer A et al. Lancet. 2011.
- 122,738 infants: Zhao et al. Lancet. 2014.
- 42,169 infants: Schena F et al. J of Peds. 2017.
- 8,013 infants: Slitine N et al. Int J Neonatal Screen. 2020..
- 8,305 infants: Zhang et al. Int J Gen Med. 2021.
All studies using Masimo SET® concluded that pulse oximetry, in conjunction with clinical assessment, improved screening sensitivity when compared to clinical assessment alone.2,3,5-14
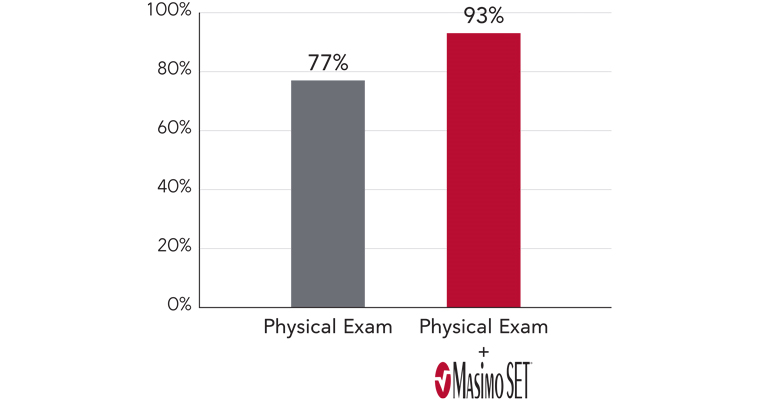
When combined with clinical assessment, Masimo SET® improved CCHD screening sensitivity to 93%2
Improved CCHD Detection vs. Physical Exam Alone
In a study of 122,738 infants — the largest CCHD screening study to date — Masimo SET® improved CCHD screening sensitivity to 93% when combined with clinical assessment. In addition, 46% of babies with false positive screening results needed medical intervention or further monitoring due to other abnormalities including pulmonary hypertension, lung problems, and other CHDs.2

Including Perfusion Index Measurement in CCHD Screening
Studies have shown that incorporating perfusion index (Pi) into screening may increase sensitivity to the detection of CCHD in infants.21,22 In a study of 10,009 infants, when Pi was added to CCHD screening, screening revealed abnormalities in 100% of all newborns who had left heart obstructive disease.6 In another study of 66 pre-term neonates, clinicians showed that the difference in Pi values in the right hand and right leg obtained by pulse oximetry had diagnostic value in hemodynamically significant Patent Ductus Arteriosus.23
Solutions for CCHD Screening with Masimo SET® Pulse Oximetry
Solutions for CCHD Screening with Masimo SET® Pulse Oximetry
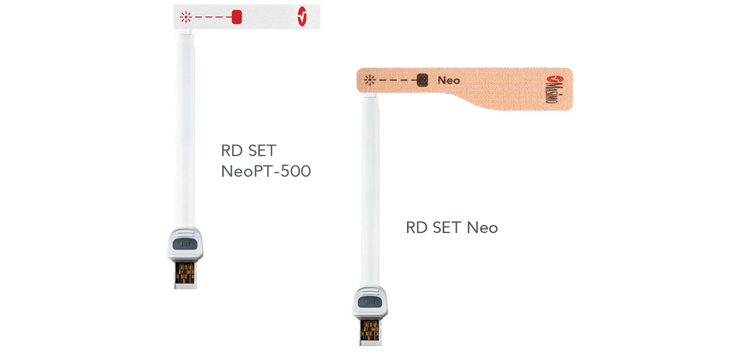
Improved Accuracy and Comfort with RD SET®
Oxygen saturation (SpO2) accuracy specifications have improved to 1.5% ARMS for all patients, including neonates < 3 kg, in conditions of motion and no motion when using RD SET sensors.* In addition, a flat, lightweight design and low-profile internal components allow gentle application on newborns during CCHD screening.
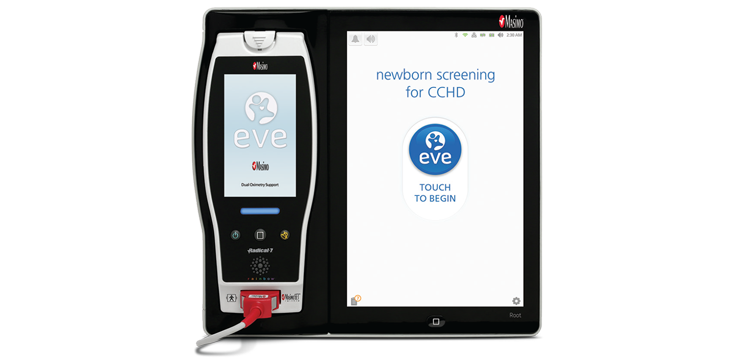
Automating Workflows with Eve™ Newborn Screening Application
Eve combines Masimo SET® measure-through motion pulse oximetry with guided workflows to standardise CCHD screenings. This application also eliminates the need for manual calculations and documentation during CCHD screenings and automates hospital CCHD screening protocols to increase consistency and improve compliance with screening guidelines.
Increased Efficiency using Dual SET Oximetry with Root
The Root platform, when used with a Masimo SET® Module, Radical-7®, and Eve, allows clinicians to obtain simultaneous measurements from two application sites using one convenient display. By enabling data transfer to hospital electronic medical records (EMRs), including CCHD screening measurements and results, Root with Masimo Hospital Automation™ also helps reduce the possibility of reporting errors.

Recommendations for CCHD Screening with Pulse Oximetry
Recommendations for CCHD Screening with Pulse Oximetry
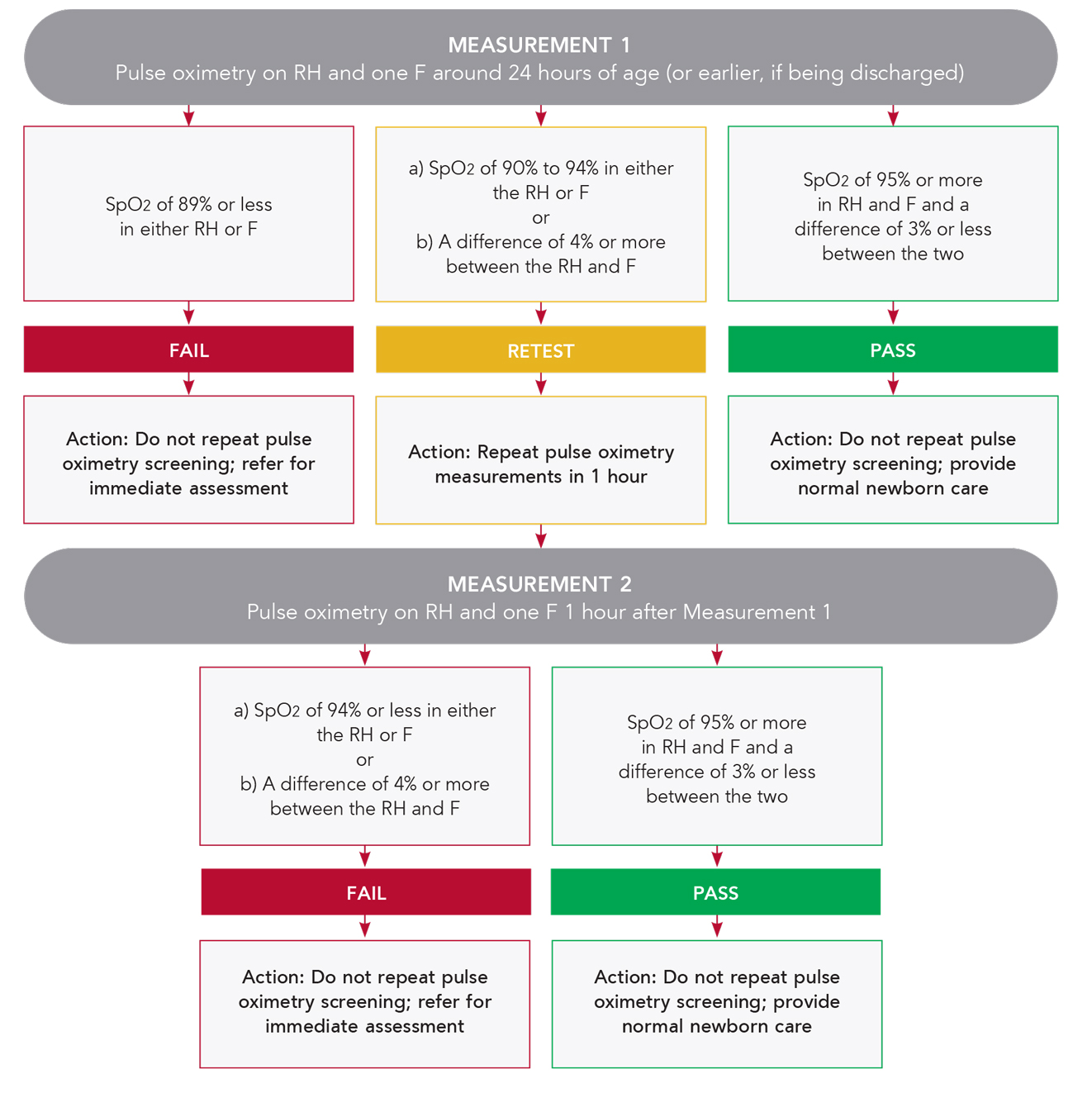
CCHD screening using pulse oximetry can be easily implemented following protocol recommendations from a workgroup of key experts and stakeholders. CCHD screening is performed by taking a preductal SpO2 measurement on the right hand (RH) and a postductal SpO2 measurement on one foot (F). Based on the automated measurement results, a pass or fail screening result is identified.
Eve Newborn Screening Application can be customised to use of the protocol shown below or others, at the discretion of the hospital or organisation.
A Workgroup Recommended CCHD Screening Protocol16
Learn More About CCHD Screening with Masimo SET®
Learn More About CCHD Screening with Masimo SET®
References:
- 1.
Ewer AK, et al. NIHR Health Technology Assessment Programme: Executive Summaries. 2012.
- 2.
Zhao et al. Lancet. 2014 Aug 30;384(9945):747-54.
- 3.
de-Wahl Granelli A et al. BMJ. 2009;Jan 8;338.
- 4.
Manzoni P, et al. Lancet Child Adolesc Health. 2017;1(2):88-90.
- 5.
Meberg A et al. J Pediatr. 2008;152:761-5.
- 6.
Schena F et al. J Pediatr. 2017;183:74-79.
- 7.
Ewer AK et al. Lancet. 2011 Aug 27;378(9793):785-94.
- 8.
de-Wahl Granelli A et al. Acta Paediatr. 2007 Oct;96(10):1455-9.
- 9.
Zhang, Yu-Lin et al. Int. J Gen Med. 2021;14:2599-2609.
- 10.
Slitine N et al. Int J Neonatal Screen. 2020;6(53).
- 11.
Gunaratne CR et al. Sri Lanka J Child Health. 2021;50(1):04-11.
- 12.
Jawin V et al. PLoS One. 2015;10(9):e0137580.
- 13.
Hamilçıkan S, Can E. J Perinat Med. 2018;46(2):203-207.
- 14.
Gopalakrishnan S et al. Med J Armed Forces India. 2021;77(2):214-219.
- 15.
Kemper AR et al. Pediatrics. 2011 Nov;128(5):e1259-67.
- 16.
Martin et al. Pediatr. 2020;146(1):e20191650.
- 17.
Abouk R et al. JAMA. 2017;318(21):2111-2118.
- 18.
Glidewell et al. MMWR Morb Mortal Wkly Rep 2019. 2019;68;107-111.
- 19.
Published clinical studies on pulse oximetry and the benefit of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- 20.
Shah N. et al. J Clin Anesth. 2012 Aug;24(5):385-91.
- 21.
Siefkes H, et al. Am J Perinatol. 2020; 37(2):158-165.
- 22.
Uygur O et al. Pediatr Neonatol. 2019;60(1):68-73.
- 23.
Buyukeren, M. et al. J Clin Neonatol. 2021;10(1):11-18.
*ARMS accuracy is a statistical calculation of the difference between device measurements and reference measurements. Approximately two-thirds of the device measurements fell within ± ARMS of the reference measurements in a controlled study.
RESOURCES

SET® Module and Eve have obtained CE Marking. Not available in the U.S.
SET® Module and Eve are not licensed for sale in Canada.
For professional use. See instructions for use for full prescribing information, including indications, contraindications, warnings, and precautions.
PLCO-005532/PLM-11167C-0122



